ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 1
Efficacy and safety of allergopharma house dust mite vaccine in the treatment of allergic rhinitis.
1Department of Allergy, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
2Reparative and reconstructive Sugery, Beijing Shijitan Hospital, Capital Medical University Beijing, China
3Central Laboratory, The Luhe Teaching Hospital of the Capital Medical University, Beijing, China
4Plastic Surgery, The Luhe Teaching Hospital of the Capital Medical University, Beijing, China
- *Corresponding Author:
- Xueyan Wang
Department of Allergy
Beijing Shijitan Hospital
Capital Medical University
Beijing, China
Accepted date: October 07 2014
Allergic rhinitis (AR) is a common, frequently occurring disease with increasing incidences every year. House dust mite is one of the common indoor allergens that causes allergic rhinitis. So this study was aimed to evaluate the efficacy and safety of Allergopharma house dust mite vaccine in the treatment of allergic rhinitis. A case self-control method was used to compare the total symptom using visual analog scale (VAS), nasal symptom score, as well as medication changes in 68 cases of patients with house dust mite-induced allergic rhinitis before and after receiving one year of immunotherapy. Out of 68 patients, 55 completed immunotherapy. After treatment, the total symptom VAS and nasal symptom score of 55 patients were significantly reduced compared with before treatment (P<0.01), and medication dosage was also lowered. Among a total of 1683 injections, local adverse reactions appeared 134 times, and mild systemic adverse reactions 17 times. However, no fatal systemic adverse reaction was observed. Allergopharma house dust mite vaccine is an effective and safe method of treatment for house dust mite-induced allergic rhinitis.
Keywords
House dust mite, Allergic rhinitis, Immunotherapy, Allergopharma house dust mite vaccine
Introduction
Allergic rhinitis (AR) is a frequently occurring disease with increasing incidences every year [1]. Therapeutic principles of AR include avoidance of contact with allergens, pharmacological control of symptoms, allergen specific immunotherapy (SIT), and propaganda and education. SIT is the only means of etiological treatment recommended by the World Health Organization (WHO), whose efficacy has been fully confirmed; SIT also requires the use of standardized allergen vaccines [2]. Allergopharma house dust mite vaccine is a standardized allergen vaccine, which is in use, overseas, for a long time. However, till date, China has relatively scant experience in its application [3,4]. This study aims to further evaluate its efficacy and safety when applied to Chinese population, and to explore its maximum tolerated dose during the maintenance treatment phase.
Materials and Methods
Clinical case data
All the cases were from outpatients of the Department of Allergy, Beijing Shijitan Hospital affiliated to Capital Medical University between April 2010-April 2012.
Inclusion criteria
① Age 5-55 years (male or female). ② Patients clearly diagnosed with AR according to the 2009 "Guidelines for Diagnosis and Treatment of Allergic Rhinitis" criteria [5]. ③ House dust mite exposure-related clinical history. ④ Allergen detection results (within 3 months before SIT) which satisfy the following two items: dermatophagoides pteronyssinus and/or dermatophagoides farinae skin prick test (SPT) ≥ grade 2. Serum sIgE detection: dermatophagoides pteronyssinus slgE ≥ 0.7 KU/L and/or dermatophagoides farinae slgE ≥ 0.7 KU/L. ⑤ Signing of informed consent.
Exclusion criteria
① Complicated with asthma. ② Presence of sensitivity to other seasonal or perennial allergens in addition to dermatophagoides pteronyssinus and dermatophagoides farinae, except for avoidable pets (until not in contact with pets). ③ VAS ≤ 3. ④ SIT contraindications: such as severe immunological diseases, major cardiovascular diseases, cancers and chronic infections, and severe hepatic and renal metabolic diseases. Co-administration of β- blockers (including eye drops), or ACE inhibitors, etc. ⑤ Women preparing for pregnancy, or already pregnant or lactating. ⑥ Patients who had received SIT in the past. ⑦ Subjects participated in other studies within 30 days prior to enrollment.
Elimination criteria: Patients want to quit therapy, breach of protocol, loss of follow-up.
Determination methods
Skin prick test solution from Allergopharma, Germany was used in the SPT, and Pharmacia CAP system was used in the determination of serum sIgE; operation and judgment of results were done in strict accordance with requirements.
Immunotherapy
In this study, mite allergen injection (Novo-Helisen® Depot: 50% dermatophagoides pteronyssinus + 50% dermatophagoides farinae) manufactured by Allergopharma, Germany was injected subcutaneously to the patients, the concentration unit TU/ml (therapeutic unit) of the preparation was categorized into grades 0, 1, 2 and 3, which corresponded to the concentrations (5, 50, 500 and 5000) TU/ml; initial therapy (dose escalation phase): began with the lowest concentration (grade 1 or 0) of the minimum dose, for children and highly sensitive patients, the therapy began with grade 0, injection interval was 7~14 days during the dose escalation phase. Dose was escalated to the individual’s maximum tolerance. If the last dose was not well tolerated, the same or a lower dose was used; generally, each concentration increased with escalating doses of 0.05, 0.1, 0.2, 0.4, 0.6 and 0.8 mL until grade 3 was reached, therapy was maintained at grade 3 (dose maintenance phase), the dose was the maximum tolerated dose for each patient, which differed among patients, and did not exceed 1.0 mL of grade 3 concentration, injection was given at an interval of once every 4~6 weeks during the maintenance therapy phase. Total duration of therapy is recommended to be not less than three years. However, in this study, efficacy was observed for only one full year.
Pharmacotherapy
At the beginning of or during the immunotherapy, the use of anti-allergic drugs (such as antihistamines, glucocorticoids, leukotriene antagonists) was allowed to be continued. The type, dose, administration method and frequency of anti-allergic agents were adjusted according to the changes in patients' conditions.
Efficacy evaluation
Symptoms and medication status of patients were acquired and recorded during visit 1 (on the day SIT was initiated), visit 2 (6 months ± 14 days from the initiation of SIT) and visit 3 (12 months ± 14 days from the initiation of SIT), more specifically.
Primary efficacy indicators
① VAS, a 10-cm long line, where the left endpoint represents no symptom, and the right endpoint represents the greatest clinical symptom distress, patients were asked to mark a point on the line by themselves based on the severity of clinical symptoms, and then the researchers measured the linear distance from the left endpoint to the marked line using the ruler as the VAS score. ② Nasal symptom score: Symptom scores of patients were recorded every day within two weeks prior to visits 1, 2 and 3, and the total daily symptom score was informed to the researchers on the day of visit, scoring items include nasal obstruction, nasal itching, sneezing and nasal discharge, and each item was scored on a 0~3 scale (0 = no symptom, 1 = mild, 2 = moderate, 3 = severe) [6,7].
Based on previous literature, patients whose scores of the above two symptoms improved over 30% after therapy were considered to be clinically significant [8].
Secondary efficacy indicators
Medication status; the types, names, dosage, administration methods and frequency of anti-allergic agents were recorded for each patient during visits 1, 2 and 3; and changes in medication status in patients before and after treatment were compared.
Assessment of adverse reactions
Patients were observed for at least half an hour after each injection and before the next injection of mite allergen to assess drug-related adverse reactions. Adverse reactions were categorized based on the severity into: ① Local reactions—( a) mild local reactions; skin rash with a diameter less than 4 cm, itching, subsiding within 24 hours, and (b) strong local reactions; skin rash with a diameter greater than 4 cm (redness, itching, irritation, pseudopodia), which lasted more than 24 hours, and ② Systemic reactions: systemic adverse reactions were graded using the assessment criteria established in the ARIA guidelines (2008). Grade 0: no symptoms or non-specific symptoms; grade I: mild systemic reactions, symptoms of localized urticaria, rhinitis or mild asthma (peak flow < 20% decrease from baseline); grade II: moderate systemic reactions, symptoms of slow onset of generalized urticaria, and/or moderate asthma (peak flow < 40% decrease from baseline); grade III: severe (non-life-threatening) systemic reactions, symptoms of rapid onset (< 15 min) of generalized urticaria, angioedema, or severe asthma (peak flow > 40% decrease from baseline); grade IV: anaphylactic shock, symptoms of immediate evoked reaction of itching, flushing, erythema, generalized urticaria, angioedema, immediate asthma, hypotension, etc.
Statistical analysis
SPSS 11.0 statistical software was used, measurement data were expressed as x ±s, and compared by the t test, count data were expressed as percentages, and compared by the x2 test, P<0.05 was considered statistically significant.
Efficacy and safety of Allergopharma
ResultsResults
Enrollment and completion status
A total of 68 patients were enrolled, including 31 males and 37 females aged between 7-55 years with a mean ageof 33.4 years, and had 1-26 year/s of medical history, with a mean value of 4.82 years; SPT mite allergen positive (++) / (+++) / (++++) 11/37/20; mite-specific IgE (KU/L) 2.32 (0.73~85.60). A total of 55 patients completed the full course of treatment which lasted for one full year. One patient quitted the therapy due to unsatisfactory efficacy, another patient withdrew from the therapy because of local reactions, 5 patients quitted the therapy due to the time and inability to stick to the injections, and 6 patients were lost during follow-up. Efficacy was not evaluated when the course of therapy was less than one year, hoever, safety evaluation was still performed.
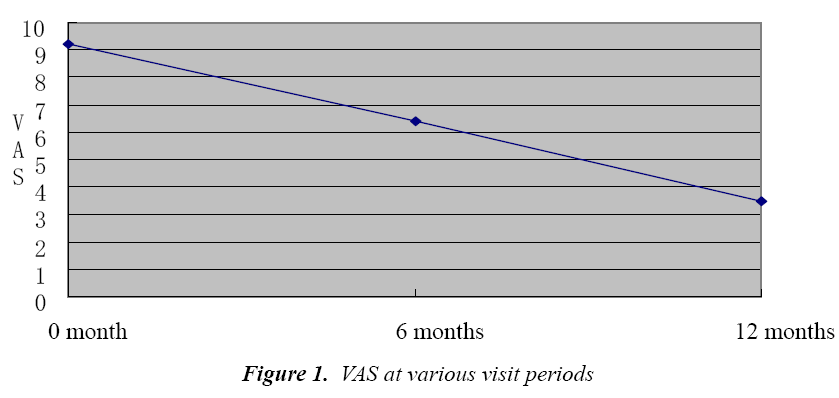
VAS score
It can be seen that during the immunotherapy, VAS score of patients showed a decreasing trend, which was particularly evident after 1 year, (P<0.01, Fig. 1). Efficacy evaluation after one year showed that among 55 patients, 47 patients had over 30% reduction in total symptom scores than before, which suggests an efficacy rate of 85.46%.
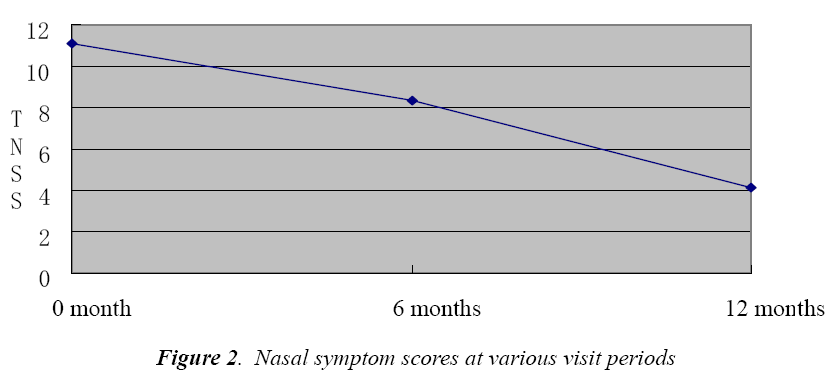
Nasal symptom score
As can be seen, the total nasal symptom score (TNSS) of patients also showed a decreasing trend along with the course of therapy (P<0.01, Fig. 2). One year later, efficacy was calculated based on the nasal symptom score, 45 patients had over 30% reduction in symptom score than before, so the efficacy rate was 81.81%.
Medication changes
One year later, among 55 patients, medication was reduced in 49 cases, unchanged in 6 cases, and increased in 0 case; medication reduction rate was 89.09%.
Safety evaluation
During 1 year of therapy and observation, a total of 1683 injections were given, and 134 times of local reactions occurred, most of which were immediate reactions. Among them, 6 patients had 19 times of severe local reactions, 2 of them showed severe local reactions when 0.6- 0.8 ml of grade 2 concentration was injected at the initial phase of therapy, and 4 had severe local reactions when 0.6-0.8 ml of grade 3 concentration was injected at the initial phase of therapy. The rest were all mild local reactions. Four patients had 17 times of systemic adverse reactions when 0.6-1.0 ml of grade 3 concentration was injected at the initial phase of therapy, of which 12 were immediate reactions, and 5 were delayed reactions, the incidence of systemic adverse reactions was 1.01%. Among them, grade I reactions appeared 15 times, and grade II reactions 2 times, no grade III or IV adverse reactions occurred. Symptoms were relieved immediately by reducing the dosage or pharmacotherapy in all cases with adverse reactions.
Discussion
SIT has more than 100 years of history of clinical application, its efficacy has long been confirmed; developing from the original crude allergen extracts to the present standardized vaccines, the aim is to ensure the efficacy as far as possible while reducing treatment-related adverse reactions. House dust mite is one of the most important allergens that causes allergic diseases, so the study of Novo Helisen Depot, one of its standardized vaccines, is of important significance [3,4]. We conducted a one-year study taking patients' VAS score, total nasal symptom score and medication dosage as efficacy evaluation indicators, all of which were significantly improved compared with before treatment. VAS score of patients has been confirmed by several studies as an easy and simple method of evaluating SIT [9-11]. Hence the efficacy of Novo Helisen Depot is worthy of recognition. Out of all the adverse reactions observed and recorded, the majority were local reactions. The proportion of systemic adverse reactions was relatively low, and their severity was also relatively mild, all of which were alleviated after reduction of dosage or symptomatic treatment. All adverse reactions, especially systemic adverse reactions; which occurred mostly within 30 minutes after injection, could be treated timely. This was found to be consistent with the literature [12,13]. It is worth noting that there were indeed some patients whose systemic adverse reactions were manifested as delayed reactions, so in the clinical settings, the dosage can still be specifically selected for individual patients. This study also showed a significant association between the incidence of adverse reactions and the injection dose, when the patients were injected with grade 3 concentration, the incidence of adverse reactions increased; which is consistent with similar studies at home and abroad [14-20]. Whether other factors such as age and gender are associated with the incidence of systemic adverse reactions was not analyzed due to too small number of subjects. This however needs to be investigated in the future. The efficacy of immunotherapy is correlated with the dose; low-dose immunotherapy is ineffective, while excessively high dose may result in unacceptable severe systemic reactions. Therefore, the ideal dose is defined as the allergen vaccine dose that can induce clinical efficacy in most patients without causing unacceptable side effects. The exploration of appropriate maintenance dose for Chinese patients is very important. As the number of cases in this study is relatively small, we could not come to a firm conclusion. Similar studies done previously at home maintained the dose up to 0.8 ml at grade 3. We found that the majority of the cases could be injected with maintenance dose up to 1.0 ml of grade 3, but after a maintenance dose of 0.6-0.8 ml of grade 3 was injected the possibility of adverse reactions increased markedly. Whether this is linked to the physical tolerance of Chinese people have to be studied in the future.
In conclusion, we believe that as one of the standardized vaccines, immunotherapy with Novo-Helisen Depot is a safe and effective method of treatment for patients with house dust mite-induced AR.
Conflict of interest if any
There is not any conflict of interest about the paper. Acknowledgement: There is not special Acknowledgement of the paper.
ReferencesReferences
- Compalati CE, Penagos M, Henley K, Canonica GW. Allergy prevalence survey by the World Allergy Organization. ACII-JWAO 2007; 19: 82-90.
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. Journal of Allergy and Clinical Immunology 1998; 102: 558-562.
- Kettner J, Meyer H, Cromwell O, Narkus A, Jost K. Specific immunotherapy with recombinant birch pollen allergen rBet v 1-FV results of 2 years of treatment (Phase II trial). 2007; Allergy, 62: 264-265.
- Ullrich D, Thum-Oltimer S, Mussler S, Jaeschke B. Successful specific subcutaneous immunotherapy (SCIT) with non-modified semi-depot pollen and mite preparations. Allergo J 2007; 16: 193-198.
- Rhinology Group, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery, Rhinology Group, Branch of Otorhinolaryngology Head and Neck Surgery of Chinese Medical Association. Chinese Journal of Otorhinolaryngology Head and Neck Surgery 2009; 12: 977-978.
- Pre-Authorisation Evaluation of Medicines for Human Use. European Medicines Agency 2008: 203-205.
- Colás C, Monzón S, Venturini M, Lezaun A. Doubleblind, placebo-controlled study with a modified therapeutic vaccine of Salsola kali (Russian thistle) administered through use of a cluster schedule. Journal of Allergy and Clinical Immunology 2006; 117: 810-816.
- Malling HJ. Immunotherapy as an effective tool in allergy treatment. Allergy 1998; 53: 461-472.
- Bousquet J, Khaltaev N, Cruz AA. Allergic Rhinitis and its Impact on Asthma (ARIA). Allergy 2008; 63: 8-160.
- Senti G, Vavricka BM, Graf N, Johansen P, Wüthrich B, Kündig TM. Evaluation of visual analog scales for the assessment of symptom severity in allergic rhinoconjunctivitis. Ann Allergy Asthma Immunol 2007; 98: 134-138.
- Bousquet PJ, Combescure C, Neukirch F, Klossek JM, Mechin H, Daures JP, Bousquet J. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy 2007; 62: 367-372.
- Chen JJ, Xiang JS, Kong WJ, Su H, Shi QM. A Luoge desensitization treatment of allergic rhinitis safety studies. Journal of Clinical Otorhinolaryngology 2006; 20: 208-209.
- Qu SH, Li TY, Xu G, Wen WP, Shi JB, Lin ZB, Chen YQ. Immunotherapy in allergic rhinitis and its impact on the dynamic assessment of the impact of asthma. Journal of Sun Yat-sen University (Medical Sciences) 2006; 27: 575-578.
- Qu SH, Li TY, Xu G, Wen WP, Shi JB, Lin ZB, Chen YQ. Immunotherapy in allergic rhinitis and its impact on the dynamic assessment of the impact of asthma. Journal of Sun Yat-sen University (Medical Sciences) 2006; 27: 575-578.
- Xiang L, Shen KL, Zhang HY, He JX, Zhao JM. Asthmatic children on standardized dust mite immunotherapy tolerated dose escalation phase. Chinese Journal of Practical Pediatrics 2006; 21: 924-926.
- Wang HY, Lin XP, Hao CL, Zhang CQ, Sun BQ, Zheng JP, Chen P, Sheng JY, Wu YY, Zhong NS. Standardized house dust mite immunotherapy vaccine efficacy against allergic bronchial asthma Journal of Tuberculosis and Respiratory Diseases. Chinese Journal of Tuberculosis and Respiratory Diseases 2006; 29: 679-687.
- Xiao ZA, He XB, Wu WJ, Yang S, Tian JY, Yang CY, Xie DH, Huang BY. A Luoge allergen skin test and specific desensitization in the diagnosis and treatment of allergic rhinitis significance. Chinese Journal of Otorhinolaryngology-skull Base Surgery 2007; 13: 212-215.
- Wang KP, Wang X, Yin KS. Dust mite allergen vaccine subcutaneous treatment of allergic asthma. Journal of Nanjing Military Medical College 2003; 25: 237-239.
- Huang LJ, Deng XH, Ruan J, Liu YZ. Mixed mite treatment of children with allergic rhinitis vaccine efficacy. Chinese Journal of Asthma (Electronic Version) 2009; 3: 20-22.
- Shi HY, Wang XY, Ren HL, Zhuang Y. Dust mite allergen specific immunotherapy of chronic eczema efficacy analysis. The Chinese Journal of Dermatovenereology 2010; 24: 424-426.
- Wu QR, Lai H, Zou Y. Allergen immunotherapy for allergic rhinitis and its mechanisms. Qinghai Medical Journal 2006; 36: 2-4.