ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 10
Effects of enzymatic digestion, cell culture and preservation conditions on surface CD62L expression of primary murine CD3+CD4+ T cells
1State Key Laboratory of Virology and Medical Research Institute, Hubei Province Key Laboratory of Allergy and Immunology and Department of Immunology, Wuhan University School of Medicine, Wuhan, PR China
2The Eighth Hospital of WUHAN, Wuhan, P.R China
- *Corresponding Author:
- Qin Pan
State Key Laboratory of Virology and Medical Research Institute
Hubei Province Key Laboratory of Allergy and Immunology
and Department of Immunology
Wuhan University School of Medicine, PR China
Accepted on April 9, 2018
DOI: 10.4066/biomedicalresearch.29-17-3070
Visit for more related articles at Biomedical ResearchSurface markers are used to identify distinct cell populations. CD62L, a cell adhesion molecule on leukocytes, is used to identify and segregate central memory T (TCM) and effector memory T (TEM) cells. In the present study, we evaluated the effects of experimental conditions on surface CD62L expression of primary murine CD3+CD4+ T cells. We found that CD62L expression levels were more resistant to collagenase D treatment. Prolonged culture (at 37°C) and preservation (at 4°C) periods resulted in modulation of surface CD62L expression in vitro. These results provide valuable information for the development and optimization of a reliable method for detecting of CD62L expression in vitro.
Keywords
CD62L, Memory T cells, Flow cytometry.
Introduction
CD62L, also known as L-selectin, is a cell adhesion molecule that is constitutively expressed on leukocytes [1]. CD62L acts as a homing receptor and is involved in leukocyte homing to peripheral lymph nodes, which contributes to the initiation and maintenance of immune responses to pathogens [2]. CD62Lknockout mice exhibit a severe reduction in the number of lymphocytes homing to peripheral lymph nodes [3]. CD62L is found on the surface of naïve T cells, which need to enter secondary lymph nodes to encounter their target antigen [4]. Rapid down-regulation of CD62L on activated T cells has been shown to prevent their re-entry into peripheral lymph nodes [5]. More importantly, distinct subpopulations of memory T cells have been described and are recognized based on their differential expression of CD62L [6]. Central memory T (TCM) cells, which are located in secondary lymphoid organs, are characterized by their surface CD62L expression and CD45RO/CD44 activation marker in humans and mice [6,7]. Effector memory T cells (TEM), which are thought to confer immediate effector function in peripheral tissues, have lost the homing receptor CD62L [6]. It has been reported that surface CD62L expression can dramatically change within minutes, even during standard laboratory procedures such as Ficoll- Hypaque/Percoll density gradient centrifugation and storage at room temperature for more than 4 h [8,9]. These findings suggest that CD62L expression might be sensitive to experimental conditions. In the present study, we evaluated the effects of enzymatic digestion, cell culture and preservation conditions on surface CD62L expression on primary murine CD3+CD4+ T cells. These findings provide valuable information for the development and optimization of a reliable method for detecting CD62L expression.
Materials and Methods
Animals
Six to eight week-old female C57BL/6 mice (the Animal Laboratory Center, Wuhan University) were fed a commercial pellet diet and provided water ad libitum. The mice were housed under standard laboratory conditions of temperature (22°C), humidity (40-60%), and lighting (12 h light/dark cycle). Animal maintenance, care and use were performed in compliance with all guidelines and approved by the Institutional Animal Care and Use Committee of Wuhan University.
Lung and spleen collection and lymphocyte preparation
Mice were sacrificed and placed in dorsal recumbency. The spleen and lung tissues were collected. The spleens were homogenized as previously described [10]. The connective tissues and other debris were removed with a nylon mesh filter. After the red blood cells were lysed with Tris-buffered ammonium chloride, the splenocytes were cultured (2 × 106 cells per ml) in a humidified atmosphere with 5% CO2 at 37°C or preserved at 4°C in RPMI-1640 tissue culture medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA), 10 mM L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin. In the enzyme digestion group, the splenocytes were incubated with collagenase I, IV, V or D for the indicated time periods at 37°C. All collagenases were derived from Clostridium histolyticum (Sigma-Aldrich, St. Louis, MO, USA). After washing, the cells were collected for Flow Cytometry (FCM) analysis. To prepare single-cell suspensions from murine lung tissue, three different procedures were used. In the mechanical dissociation group, the lungs were perfused with 10 ml Phosphate-Buffered Saline (PBS) through the right ventricles of the hearts before removal. The lung tissue was then gently dissociated over a sterile, metallic sieve screen. Once the tissue was dissociated, the cells were filtered through a nylon mesh filter to remove the connective tissue matrix. Next, the cells were washed and seeded into cell culture plates. In the enzyme digestion group, lung tissue was cut into small pieces and incubated with 5 ml of collagenase I, IV, V or D (1 mg/ml in PBS) for 10 min at 37°C. In the broncho-alveolar lavage fluid (BALF) group, the murine lung was lavaged three times with 0.5 ml of PBS containing 10 % fetal bovine serum. The retained BALF was centrifuged at 300 Xg for 5 min at 4°C to collect cell pellets. The BALF cells were resuspended in 200 μl of PBS for FCM analysis.
FCM analysis
The cells were collected and washed with PBS and, then stained with APC anti-mouse CD3 (clone: 145-2C11, Biolegend, San Diego, CA, USA), FITC anti-mouse CD4 (clone: RM4-5, Biolegend) and PerCP anti-mouse CD62L (clone: MEL-14, Biolegend). Propidium Iodide (PI) staining was used in FCM analysis to exclude dead cells and assess cell viability. During FCM analysis, 2 μg/ml of fluorescent-labeled antibody (anti-CD3, anti-CD4 and anti- CD62L antibodies) was incubated with 1 × 106 cells in 100 μl of FACS Buffer (PBS, 0.5-1 % bovine serum albumin) for 30 min at room temperature according to the manufacturer’s instruction. The cell suspensions were analysed with a BD Accuri™ C6 Plus flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Gates were set based on the staining profile of the isotype control. The data were analysed using FlowJo software (version 7.6.3, Ashland, OR, USA).
Statistics analysis
The data were analysed with SPSS software (SPSS, Inc., Chicago, IL USA). Differences were considered statistically significant for p-values<0.05. Experimental data were analysed by analysis of variance.
Results
CD62L expression of splenic CD3+CD4+ T cells is more resistant to collagenase D treatment than to collagenases I, IV and V treatment
Several studies showed that collagenase-based digestion procedures resulted in changes in the expression of some molecules on the leucocyte cell surface [11,12]. Here, we assessed the effects of collagenases I, IV, V and D digestion on the surface CD62L expression of splenic CD3+CD4+ T cells.
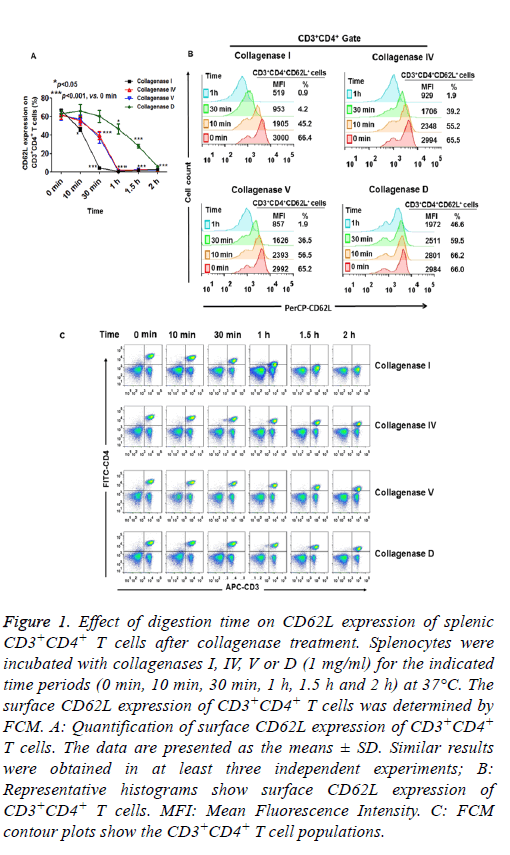
As expected, incubation of dissociated tissue with collagenases (I, IV, V and D) for 2-4 h resulted in reduced cell viability (data not shown). Therefore, we measured surface CD62L expression within 2 h of collagenase treatment. As shown in Figure 1A and 1B, when 1 mg/ml of collagenases were used, CD62L expression of splenic CD3+CD4+ T cells was significantly reduced after 10 min digestion with collagenase I. CD62L expression did not change when the cells were incubated with collagenase IV and V for 10 min, while 30 min treatment with collagenases IV and V caused a significant reduction in CD62L expression (Figures 1A and 1B). Only collagenase D had a minor effect on CD62L expression within 30 min of digestion (Figures 1A and 1B). After 1 h digestion, the CD62L expression levels of splenic CD3+CD4+ T cells were greatly reduced in all collagenase-treated groups (Figures 1A and 1B). These results indicated that CD62L expression of CD3+CD4+ T cells was more resistant to collagenase D treatment than to collagenases I, IV and V treatment.
Figure 1: Effect of digestion time on CD62L expression of splenic CD3+CD4+ T cells after collagenase treatment. Splenocytes were incubated with collagenases I, IV, V or D (1 mg/ml) for the indicated time periods (0 min, 10 min, 30 min, 1 h, 1.5 h and 2 h) at 37°C. The surface CD62L expression of CD3+CD4+ T cells was determined by FCM. A: Quantification of surface CD62L expression of CD3+CD4+ T cells. The data are presented as the means ± SD. Similar results were obtained in at least three independent experiments; B: Representative histograms show surface CD62L expression of CD3+CD4+ T cells. MFI: Mean Fluorescence Intensity. C: FCM contour plots show the CD3+CD4+ T cell populations.
Unlike the effects of collagenase treatment on CD62L expression, there were only minor changes in the expression of CD4 and CD3 on the cells within 30 min of collagenase digestion (I, IV, V and D, Figure 1C). However, the proportion of splenic CD4+CD3+ T cell was greatly decreased after 1 or 1.5 h digestion in the collagenases I, IV and V treatment groups, because of the substantial reduction in CD4 expression in the cells. CD4 expression was slightly reduced when the cells were treated with collagenase D for 2 h (Figure 1C). These results suggested that different surface molecules had varying sensitivities to different collagenase digestions.
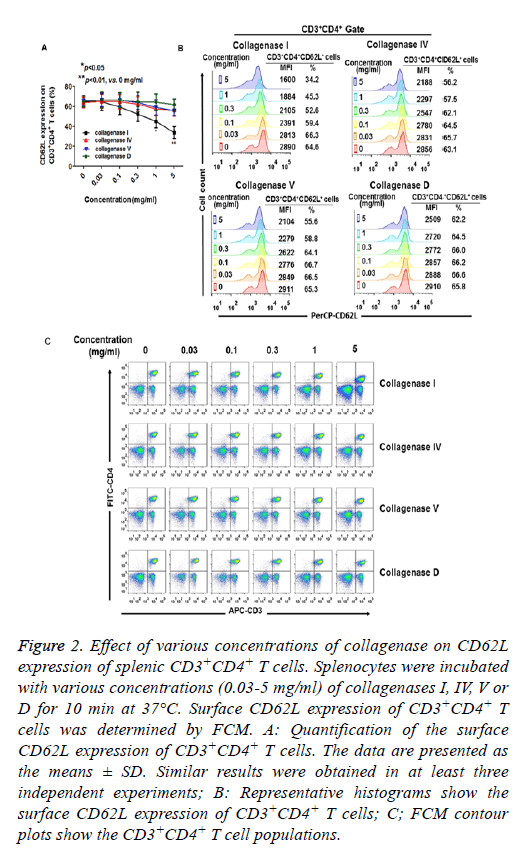
The effects of various concentrations of collagenase on CD62L expression were also assessed. As shown in Figures 2A and 2B, when the cells were incubated with collagenases for 10 min, CD62L expression was significantly reduced as the concentration of collagenase I was increased to 1 mg/ml. CD4 expression was also reduced when the cells were treated with 5 mg/ml of collagenase I. However, increased collagenases IV, V and D had only minor effects on the expression of CD62L, CD3 and CD4 during 10 min digestion (Figures 2A-2C).
Figure 2: Effect of various concentrations of collagenase on CD62L expression of splenic CD3+CD4+ T cells. Splenocytes were incubated with various concentrations (0.03-5 mg/ml) of collagenases I, IV, V or D for 10 min at 37°C. Surface CD62L expression of CD3+CD4+ T cells was determined by FCM. A: Quantification of the surface CD62L expression of CD3+CD4+ T cells. The data are presented as the means ± SD. Similar results were obtained in at least three independent experiments; B: Representative histograms show the surface CD62L expression of CD3+CD4+ T cells; C; FCM contour plots show the CD3+CD4+ T cell populations.
CD3+CD4+ T cells are highly enriched in the murine lung parenchyma
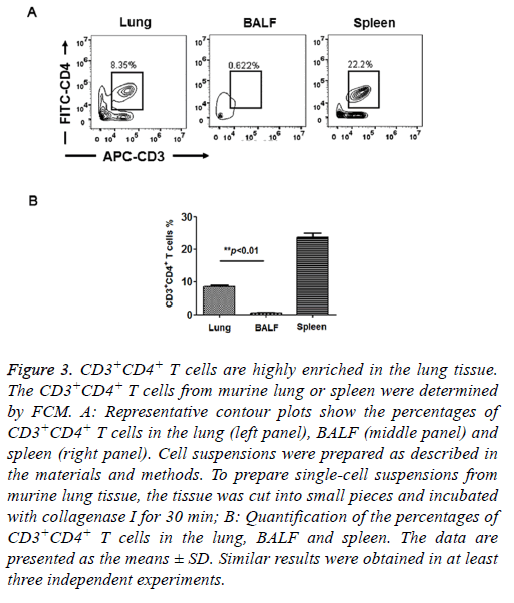
T cells isolated from peripheral tissues have been widely used to evaluate adaptive immune response in situ. Therefore, we assessed the distribution of CD4+ T cells in murine lungs. We prepared cell suspensions from lung tissues and BALF and determined the CD4+ T cell percentage by FCM. As shown in Figure 3, the percentage of CD3+CD4+ T cells in the total cell population was approximately 8.52 ± 0.41% in the lung, which was 16-fold higher than the percentage of CD3+CD4+ T cells in the BALF (0.53 ± 0.23%). In the control group, the percentage of CD3+CD4+ T cells was approximately 23.7 ± 1.35% in the spleen, which is consistent with the results of a pervious study [13]. Here, our results demonstrated that very few CD3+CD4+ T cells were present in the BALF. T cells were highly enriched in the murine lung tissue. Therefore, in the experiments below, we prepared CD3+CD4+ T cells from lung tissue.
Figure 3: CD3+CD4+ T cells are highly enriched in the lung tissue. The CD3+CD4+ T cells from murine lung or spleen were determined by FCM. A: Representative contour plots show the percentages of CD3+CD4+ T cells in the lung (left panel), BALF (middle panel) and spleen (right panel). Cell suspensions were prepared as described in the materials and methods. To prepare single-cell suspensions from murine lung tissue, the tissue was cut into small pieces and incubated with collagenase I for 30 min; B: Quantification of the percentages of CD3+CD4+ T cells in the lung, BALF and spleen. The data are presented as the means ± SD. Similar results were obtained in at least three independent experiments.
Ten minutes of digestion with collagenases have minor effects on CD62L expression of lung CD3+CD4+ T cells
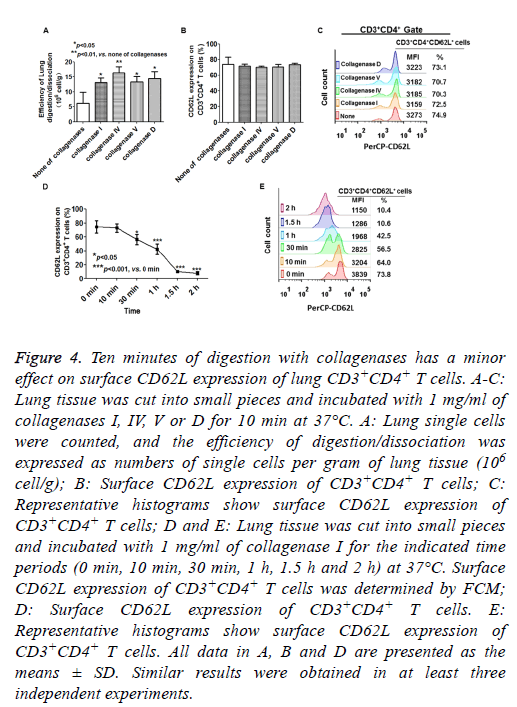
Collagenases are generally used for tissue disintegration and the isolation of lymphocytes from murine lungs. Mechanical dissection combined with collagenase digestion resulted in greater yields of viable cells compared with pulverization [14,15]. Therefore, we assessed the effects of collagenase digestion on CD62L expression on lung CD3+CD4+ T cells. As shown in Figure 4A, the lung tissue was more effectively digested and dissociated by collagenase treatment than by mechanical dissociation. The CD62L expression of lung CD3+CD4+ T cells was more resistant to collagenase I treatment compared with splenic CD4+CD3+ T cells, because the CD62L expression of lung CD3+CD4+ T cells was not significantly altered after 10 min of digestion in all collagenase treatment groups (Figures 4B and 4C). However, more than 30 min of digestion with collagenase I caused a significant reduction in CD62L expression of lung CD3+CD4+ T cells (Figures 4D and 4E). These results indicated that 10 min of digestion with collagenases (I, IV, V and D) had minor effects on CD62L expression of lung CD3+CD4+ T cells.
Figure 4: Ten minutes of digestion with collagenases has a minor effect on surface CD62L expression of lung CD3+CD4+ T cells. A-C: Lung tissue was cut into small pieces and incubated with 1 mg/ml of collagenases I, IV, V or D for 10 min at 37°C. A: Lung single cells were counted, and the efficiency of digestion/dissociation was expressed as numbers of single cells per gram of lung tissue (106 cell/g); B: Surface CD62L expression of CD3+CD4+ T cells; C: Representative histograms show surface CD62L expression of CD3+CD4+ T cells; D and E: Lung tissue was cut into small pieces and incubated with 1 mg/ml of collagenase I for the indicated time periods (0 min, 10 min, 30 min, 1 h, 1.5 h and 2 h) at 37°C. Surface CD62L expression of CD3+CD4+ T cells was determined by FCM; D: Surface CD62L expression of CD3+CD4+ T cells. E: Representative histograms show surface CD62L expression of CD3+CD4+ T cells. All data in A, B and D are presented as the means ± SD. Similar results were obtained in at least three independent experiments.
CD62L expression of CD3+CD4+ T cells is dynamic during in vitro culture at 37°C and preservation at 4°C
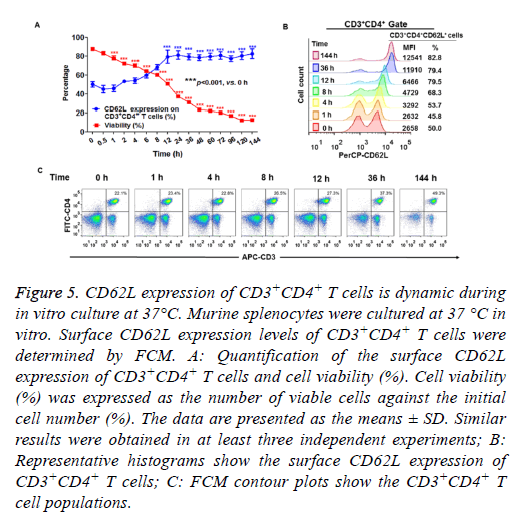
Next, we determined the surface CD62L expression of CD3+CD4+ T cells cultured in vitro. Murine splenocytes were cultured under conventional conditions (as described in the materials and methods) in humidified atmosphere with 5% CO2 at 37°C. As shown in Figure 5A, the cell viability curve progressively reduced over time. These data indicated that the total number of viable cells decreased over the culture period. However, the percentage of CD62L+CD3+CD4+ cells among viable cells gradually increased during the 2 h culture period and plateaued within 12 h in the in vitro culture period (Figures 5A and 5B). These results indicated that the prolonged in vitro culture period enriched the CD62L+ cells in the CD3+CD4+ T cell population. The percentage of CD3+CD4+ cells did not change within 36 h of in vitro culture. However, the percentage of CD3+CD4+ cells increased after 144 h of in vitro culture (Figure 5C).
Figure 5: CD62L expression of CD3+CD4+ T cells is dynamic during in vitro culture at 37°C. Murine splenocytes were cultured at 37 °C in vitro. Surface CD62L expression levels of CD3+CD4+ T cells were determined by FCM. A: Quantification of the surface CD62L expression of CD3+CD4+ T cells and cell viability (%). Cell viability (%) was expressed as the number of viable cells against the initial cell number (%). The data are presented as the means ± SD. Similar results were obtained in at least three independent experiments; B: Representative histograms show the surface CD62L expression of CD3+CD4+ T cells; C: FCM contour plots show the CD3+CD4+ T cell populations.
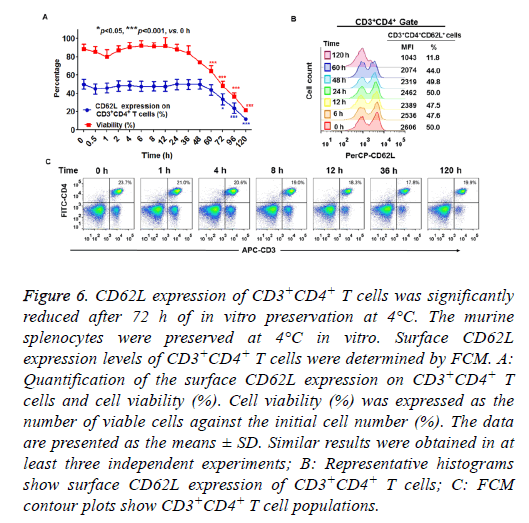
When splenocytes were preserved at 4°C in vitro, cell viability was decreased as the preservation time increased (Figure 6A). The percentage of viable cells was more than 80 % within 36 h of incubation, but was greatly decreased to 20 % after 120 h of preservation at 4°C (Figure 6A). As shown in Figures 6A and 6B, more than 48 h of preservation slightly decreased CD62L expression on CD3+CD4+ T cells. Significant differences were detected in the percentage of CD62L expression when the cells were preserved in vitro at 4°C for more than 72 h (Figures 6A and 6B). These results demonstrated that more than 72 h of preservation at 4°C led to reduced CD62L expression of CD3+CD4+ T cells. The percentage of CD3+CD4+ cells slightly decreased after 120 h preservation because of a substantial loss of these cells (Figure 6C).
Figure 6: CD62L expression of CD3+CD4+ T cells was significantly reduced after 72 h of in vitro preservation at 4°C. The murine splenocytes were preserved at 4°C in vitro. Surface CD62L expression levels of CD3+CD4+ T cells were determined by FCM. A: Quantification of the surface CD62L expression on CD3+CD4+ T cells and cell viability (%). Cell viability (%) was expressed as the number of viable cells against the initial cell number (%). The data are presented as the means ± SD. Similar results were obtained in at least three independent experiments; B: Representative histograms show surface CD62L expression of CD3+CD4+ T cells; C: FCM contour plots show CD3+CD4+ T cell populations.
Discussion
During the secondary immune response, memory T cells induce a more effective and faster immune response than the primary immune response to eliminate the infection. Some surface markers have been used to identify distinct memory cell populations and delineate various stages in these cells. CD62L, a homing receptor, is required for lymphocytes to enter secondary lymphoid tissues and initiate T-cell rolling. CD62L is highly expressed on TCM cells and is considered a typical marker for identifying both human and murine TCM cells [6,7]. TCM cells continuously recirculate via the blood stream to lymphoid organs, have a high proliferative capacity and rapidly become effectors upon re-exposure to antigens [6,16]. To date, a limitation in studies of memory T cells is that CD4 memory is far less well understood than CD8 memory, and very little is known about the optimal generation of memory T cells or kinetics of the effector to memory cell transition [17]. More importantly, the expression of cell surface markers may be influenced by experimental conditions, which makes it difficult to draw accurate conclusions based on experimental data. Thus, it is necessary to determine the effects of experimental conditions on CD62L expression of T cells, particularly CD4+ T cells.
In the present study, we evaluated the effects of enzymatic digestion on the surface CD62L expression of primary murine CD4+ T cells from both spleen and lung tissues. Our results demonstrated that CD62L expression of splenic CD3+CD4+ T cells was more resistant to collagenase D treatment than to collagenases I, IV and V treatment (Figures 1A, 1B, 2A and 2B). We found that CD62L expression did not decrease within 30 min of digestion with 1 mg/ml of collagenase D (Figures 1A and 1B). Consistent with our data, Autenberger et al. also showed that collagenase D treatment had only minor effects on the surface expression of 48 molecules tested, including CD62L [18].
The expression of CD62L of lung CD3+CD4+ T cells was more resistant to collagenase I treatment compared with that of splenic CD4+CD3+ T cells. Ten minutes of digestion with collagenase I (1 mg/ml) caused a marked loss in CD62L expression of splenic CD3+CD4+ T cells (Figures 1A, 1B, 2A and 2B), but had only minor effects on CD62L expression of lung CD3+CD4+ T cells (Figures 4B and 4C). This difference is probably related to the variations in the experimental procedures used for lymphocyte preparation and collagenase treatment. Single splenic cells were prepared and treated with collagenases, while these collagenases were added to small pieces of lung tissue to prepare single cells. Therefore, collagenases function more effectively on splenic cells than on lung cells. Collagenase treatment has been used for tissue disruption and dramatically improves lymphocyte release from tissues [11,14]. However, in studies on the function of TCM isolated from tissues, removing surface CD62L molecules during the isolation process may reduce or eliminate any observable outcome in the experiments. Therefore, less than 10 min of tissue dissociation with collagenase treatment is suitable for studying TCM cells from peripheral tissues.
Although less than 2 h of cell culture (at 37°C) and less than 72 h of preservation (at 4°C) did not result in changes in surface CD62L expression of CD3+CD4+ T cells in vitro, CD62L expression was greatly modulated by prolonged incubation at 37°C and 4°C (Figures 5A, 5B, 6A and 6B). These findings indicated that surface CD62L expression was sensitive to the extracellular environment and environmental temperature. CD62L expression on CD3+CD4+ T cells in vitro should be detected in less than 2 h (or less than 72 h) under conventional cell culture conditions (or preservation). Some published studies have reported that cryopreservation and subsequent thawing did not alter the proportion of T cell subsets, but levels of CD62L expression of CD4+ T cells were reduced on thawed cells but restored if these cells were cultured overnight after thawing [19-21]. Shete et al. reported differential modulation of phenotypic composition of HIV-infected and HIVuninfected peripheral blood mononuclear cells during cryopreservation, and they reported a significant decrease in CD62L-expressing CD8+ cells [22]. These reports indicated the lability of CD62L in response to cryopreservation [22]. Additionally, several studies have indicated that in vitro generation and maintenance of T memory-like cells require antigenic restimulation and supplementation of cytokines such as Interleukin (IL)-2, IL-7 and IL-15 during cell culture [23-26]. We will investigate the mechanisms of reduction or enhancement of surface CD62L expression under these conditions in future studies. Moreover, because of the fluctuations in CD62L expression, we suggest using a combination of several markers (CCR7, CD62L, or other markers such as CD27, CD43, and/or CXCR3) for identifying and segregating TCM and TEM cells, particularly for T memorylike cells generated in vitro [6,11,23].
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81471910, 31770145, 31221061, 81501377, 31370197 and 21572173), the National Grand Program on Key Infectious Disease (2012ZX10003002-015), the National Outstanding Youth Foundation of China (81025008), the Hubei Province Technological Innovation Special Foundation Major Project (2016ACA150), the Natural Science Foundation Project of Hunan Province (2016CFA062), the Hubei Province’s Outstanding Medical Academic Leader Program (523-276003), the Hubei Province health and family planning scientific research project (WJ2017F027), the Science and the Technology Program of Wuhan (201150530141), the Wuhan Applied Basic Research Project (2015060101010030) and the Wuhan Youth Science and technology Chen'guang plan (2016070204010127).
Disclosures
The author(s) declared no conflicts of interests.
References
- McEver RP. Selectins. Curr Opin Immunol 1994; 6: 75-84.
- Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ. The human peripheral lymph node vascular addressin. An inducible endothelial antigen involved in lymphocyte homing. Am J Pathol 1993; 143: 1688-1698.
- Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity 1994; 1: 247-260.
- Vassena L, Giuliani E, Koppensteiner H, Bolduan S, Schindler M, Doria M. HIV-1 Nef and Vpu interfere with L-Selectin (CD62L) cell surface expression to inhibit adhesion and signaling in infected CD4+ T lymphocytes. J Virol 2015; 89: 5687-5700.
- Galkina E, Tanousis K, Preece G, Tolaini M, Kioussis D, Florey O, Haskard DO, Tedder TF, Ager A. L-selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigen-activated T lymphocytes. J Exp Med 2003; 198: 1323-1335.
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22: 745-763.
- Hung CY, Castro-Lopez N, Cole GT. Vaccinated C57BL/6 mice develop protective and memory T cell responses to Coccidioides posadasii infection in the absence of interleukin-10. Infect Immun 2014; 82: 903-913.
- Stibenz D, Buhrer C. Down-regulation of L-selectin surface expression by various leukocyte isolation procedures. Scand J Immunol 1994; 39: 59-63.
- Rainer TH. L-selectin in health and disease. Resuscitation 2002; 52: 127-141.
- Klein AB, Witonsky SG, Ahmed SA, Holladay SD, Gogal RM, Link L, Reilly CM. Impact of different cell isolation techniques on lymphocyte viability and function. J Immunoassay Immunochem 2006; 27: 61-76.
- Chen Z, Chen X, Xu Y, Xiong P, Fang M, Tan Z, Gong F, Zheng F. Collagenase digestion down-regulates the density of CD27 on lymphocytes. J Immunol Methods 2014; 413: 57-61.
- Curry MP, Norris S, Golden-Mason L, Doherty DG, Deignan T, Collins C, Traynor O, McEntee GP, Hegarty JE, OFarrelly C. Isolation of lymphocytes from normal adult human liver suitable for phenotypic and functional characterization. J Immunol Methods 2000; 242: 21-31.
- Mustafa A, Gillmeister L, Hernandez WP, Larsen CT, Witonsky S, Holladay SD, Kerr RP, Ahmed SA, Santo A, Gogal RM Jr. Viability and function in lymphocytes cultured from the horse, chicken, and mouse: effects of different leukocyte enrichment techniques. J Immunoassay Immunochem 2008; 29: 370-389.
- Sauer KA, Scholtes P, Karwot R, Finotto S. Isolation of CD4+ T cells from murine lungs: a method to analyze ongoing immune responses in the lung. Nat Protoc 2006; 1: 2870-2875.
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 2011; 12: 1045-1054.
- Kushida Y, Ishida JY, Fujii M, Touma M, Hosono M. Population doublings of murine CD4(+) memory T cells during continuous antigen stimulation in vivo. Cell Immunol 2014; 292: 45-52.
- Henao-Tamayo M, Ordway DJ, Orme IM. Memory T cell subsets in tuberculosis: what should we be targeting? Tuberculosis (Edinb) 2014; 94: 455-461.
- Autengruber A, Gereke M, Hansen G, Hennig C, Bruder D. Impact of enzymatic tissue disintegration on thelevel of surface molecule expression and immune cell function. Eur J Microbiol Immunol (Bp) 2012; 2: 112-120.
- Faint JM, Tuncer C, Garg A, Adams DH, Lalor PF. Functional consequences of human lymphocyte cryopreservation: implications for subsequent interactions of cells with endothelium. J Immunother 2011; 34: 588-596.
- Florek M, Schneidawind D, Pierini A, Baker J, Armstrong R, Pan Y, Leveson-Gower D, Negrin R, Meyer E. Freeze and thaw of CD4+CD25+Foxp3+ regulatory T cells results in loss of CD62L expression and a reduced capacity to protect against graft-versus-host disease. PLoS One 2015; 10: 0145763.
- Costantini A, Mancini S, Giuliodoro S, Butini L, Regnery CM, Silvestri G, Montroni M. Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods 2003; 278: 145-155.
- Shete A, Jayawant P, Thakar M, Kurle S, Singh DP, Paranjape RS. Differential modulation of phenotypic composition of HIV-infected and -uninfected PBMCs during cryopreservation. J Immunoassay Immunochem 2013; 34: 333-345.
- Blander JM, SantAngelo DB, Metz D, Kim SW, Flavell RA, Bottomly K, Janeway CA. A pool ofcentral memory-like CD4 T cells contains effector memory precursors. J Immunol 2003; 170: 2940-2948.
- Shakya A, Goren A, Shalek A, German CN, Snook J, Kuchroo VK, Yosef N, Chan RC, Regev A, Williams MA, Tantin D. Oct1 and OCA-B are selectively required for CD4 memory T cell function. J Exp Med 2015; 212: 2115-2131.
- McDonald PW, Read KA, Baker CE, Anderson AE, Powell MD, Ballesteros-Tato A, Oestreich KJ. IL-7 signalling represses Bcl-6 and the TFH gene program. Nat Commun 2016; 7: 10285.
- Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med 2007; 204: 951-961.