ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Medical Diagnosis and Study of Biomedical Imaging Systems and Applications
Effect of ischemic preconditioning combined with ?7 nAchR agonists on limb ischemia-reperfusion lung injury in rat
Dong Jun Yu, Wei Cheng Liu, Lei Li and Shi Biao Chen*
Department of Anesthesiology, the First Affiliated Hospital of Nanchang University, Nanchang, PR China
- *Corresponding Author:
- Shi Biao Chen
Department of Anesthesiology
The First Affiliated Hospital of Nanchang University, PR China
Accepted date: June 14, 2017
DOI: 10.4066/biomedicalresearch.29-16-2130
Visit for more related articles at Biomedical ResearchObjective: To investigate the effect of ischemic preconditioning combined with α7 nAchR agonists on limb ischemia-reperfusion lung injury in rats.
Methods: Fifty healthy male Sprague-Dawley rats weighing 230-280 g were randomly divided into 5 groups: sham operation group (S, also means the control group), model group(M), model and ischemic preconditioning (IP) group, model and α7 nAchR agonists group (PNU) and model and united treatment (U) group. The model of lung injury was induced by 3 h of ischemia and 3 h of reperfusion. The arterial blood was collected at 3 h- reperfusion for blood gas analysis; The lung tissues were collected for determination of Pulmonary coefficient, malondialdehyde (MDA) content, superoxide dismutase (SOD) activity and lung tissue inflammatory cytokines (IL-6 and TNF-a). The pathological changes of lung tissues were observed under light microscope, and the pathological score of lung tissue was conducted.
Results: Compared with S group, the PaO2, pH and the activity of SOD in lung tissue of M group at 3 h reperfusion were significantly decreased, Pulmonary coefficient, MDA,IL-6, TNF-a and pulmonary pathological score of lung tissue were significantly increased (P<0.05), the difference of PaCO2 was not statistically significant (P>0.05). Compared with M group, arterial PaO2and lung SOD activity of IP group, PNU Group, U group at 3 h-reperfusion decreased, while Pulmonary coefficient, lung group, MDA,IL-6, TNF-a and pulmonary pathological score of lung tissue were significantly increased (P<0.05), there was no statistically significant difference in pH and PaCO2 between the two groups (P>0.05). Compared with IP group and PNU group, PaO2 and SOD activity of lung tissue decreased in U group at 3 h reperfusion, Pulmonary coefficient, MDA, IL-6, TNF-a, pulmonary pathology scores of lung tissue increased (P<0.05). There was no significant difference in PH and PaCO2 between the two groups (P>0.05).
Conclusion: Ischemic preconditioning and α7 nAchR agonists can attenuate limb ischemia-reperfusion lung injury in rat to a certain extent, and the combined effect of the two is better than the separate application.
Keywords
Ischemic preconditioning, α7 nAchR, Limb ischemia-reperfusion lung injury
Introduction
Limb ischemia is a common clinical pathological process, such as prolonged use of tourniquet, arterial injury or embolism, abdominal aortic aneurysm surgery, severe limb crush injury [1-4]. After recovery of blood flow, ischemia perfusion not only leads to further tissue damage, but also can cause damage to distant organs [5], especially lung tissue [6,7]. Previous studies have suggested that oxidative stress and inflammatory response play an important role in the development of limb ischemia/reperfusion (I/R)-induced lung injury [5,8,9].
Different treatments have been reported to reduce I/R-induced lung injury [10-14], including ischemic preconditioning (IP) [13,14]. IP can relieve lung injury by reducing lipid peroxidation [13], inducing expression of lung tissue heme oxygenase-1 (HO-1) and increasing endogenous antioxidant capacity in vivo [14]. Nicotinic acetylcholine receptor (nAchR) is composed of five identical or different subunits of pentamer, the central forms of an ion channel, belonging to the ligand gated ion channel receptor [15]. α7 nAchR consists of five identical α7 subunits, which is important component of the cholinergic anti-inflammatory pathway (CAP) to recognize the immunoregulatory signal of the vagus nerve and transmit it to the effector cells, activating the endogenous immunoregulatory response [16,17]. α7 nAchR agonists can simulate the vagus nerve stimulation effect to reduce inflammation by regulating the inflammatory signaling pathway [17]. Activation of α7 nAchR has been demonstrated to relieve sepsis and lung injury induced by mechanical ventilation [18,19], but whether it has protective effect on I/R-induced lung injury has not been reported. Therefore, in this study we evaluated the protective effects of IP, α7 nAchR agonists, and their combination treatment on I/R-induced lung injury.
Materials and Methods
Experimental animal
Fifty healthy male Sprague Dawley rats weighing 230-280 g were provided by Nanchang University Experimental Animal Center. The rats were fed in the animal laboratories with room temperature of 22-26°C, air humidity of 50-60% and circadian rhythm of 12:12 h. They were free for drinking water, preoperative fasting lasted 12 h. All animal experiments have been reviewed and approved by Medical Research Ethics Committee of the First Affiliated Hospital of Nanchang University.
Experimental grouping and model preparation
Fifty rats were randomly divided into 5 groups (n=10): sham operation group (S), ischemia reperfusion group(M), model and ischemic preconditioning group (IP), model with α7 nAchR agonists (PNU) Group and the united treatment group (U). All rats were weighed before the operation. The first anesthesia was intraperitoneal injection of 3% pentobarbital 40 mg/kg, the rats were in the supine position with the electric pad to keep the body temperature about 37°C. The medicine application time was 17:00 pm. Anesthesia was maintained by intraperitoneal injection of 13 mg/kg pentobarbital per hour. Limb I/R model is referred to previous reports [5], in short, ligation of the hind limb roots of rats with a rubber tourniquet caused ischemia for 3 h, then remove the rubber tourniquet for 3 h reperfusion. Rats in group S were anesthetized and injected with 1 ml of saline via tail vein at 3 h. Rats in M group were treated with limb I/R and injected with 1 ml of saline via tail vein at 3 h-ischemia. Before I/R treatment, rats in IP group were pretreated with three sets of 10 min/10 min-ischemia/ reperfusion [14], the remaining operation followed with the M group; rats in PNU group were treated with limb I/R model, and at the 3 h of ischemia, 2.4 mg/kg of α7 nAchR agonist PNU-282987 (one chemical, MedChemExpress Biotechnology, USA) dissolved in 1 ml of saline [20] was injected into the tail vein; Before I/R treatment, rats in U group were pretreated with three sets of 10 min/10 min-ischemia/reperfusion, the remaining operation as the PNU group. During the experiment process, all rats were rehydrated via the tail vein.
Arterial blood gas analysis and Pulmonary coefficient
At the end of 3 h-reperfusion, carotid arteries were dissected to take 0.5 ml of arterial blood for blood gas analysis as soon as possible (Gem Premier 3000). After intraperitoneal injection of pentobarbital (90 mg/kg), rats were anesthetized and euthanized, and the thoracic cavity was opened rapidly. The lung tissues were completely removed under sterile conditions and the bloodstains on the surface of lung tissue were blotted with filter paper for weighting. Pulmonary coefficient=the wet mass of total lung (g)/body mass (g) × 100%.
HE staining of lung tissue and scoring
After weighting, the lungs were rinsed in standard saline at 4°C. After washing the blood, the lungs were dried with filter paper. 1 × 1 × 1 cm of lung tissues of in the right lung were taken and fixed with 4% paraformaldehyde for 24 h. Then it was embedded with paraffin and cut in 5 μm thickness for HE staining. The morphological changes of lung were observed under light microscopic. The HE results of lung tissue were evaluated from four aspects: pulmonary hemorrhage or congestion, pulmonary edema, neutrophil infiltration in alveoli and blood vessel, thickness of alveolar wall. The scoring is based on the different degree: none-0 points, mild-1 point (0-25% of the visual field area), moderate-2 points (25% -50% of the visual field area), serious-3 (50% -75% of the visual field area), severe-4 points (75% -100% of the visual field area) [21], a total of 16 points.
Analysis of inflammatory cytokines and biochemical markers in lung tissue homogenate
Weigh 0.1 g of the right lung tissue, according to proportion of the weight (g): volume (ml)=1:9, 9 ml of pre-coolingsaline was added, and then lung tissue was quickly cut into pieces with the ophthalmic scissors, and prepare homogenate in ice water bath. The grinded lung tissue homogenate was centrifuged at 4000 rpm, at 4°C for 10 min, and then the supernatant was stored at -20°C. The concentration of protein in the supernatant of the homogenate was determined by BCA method. The kit was purchased from Beijing Kangwei Century Biotechnology Co., Ltd. The levels of IL-6 and TNF-α in tissue homogenate were measured by enzyme-linked immunosorbent assay (ELISA) kit. (Beijing Sizhengbai Biotechnology Co., Ltd). The activities of superoxide dismutase (SOD) and malondialdehyde (MDA) was determined by kit form Nanjing Jiancheng Biology Co., Ltd. All operating procedures follow the instructions.
Data analysis and statistics
SPSS21.0 was applied for statistical analysis, measurement data was expressed as mean ± standard deviation. The single factor analysis of variance was used for the comparison between groups, and multiple selection comparison between groups employed LSD test (Fisher's Least Significant Difference). P<0.05 means that the difference was statistically significant.
Result
Arterial blood gas analysis
Arterial blood gas analysis included PO2, PCO2 and pH, reflecting the ventilatory function in rats. As shown in Table 1, the PO2 in rat arterial blood of U group was significantly higher than that of M group, IP group and PNU group, with statistical difference (P<0.05). Compared with M group, the PO2 level of IP group and PNU group was significantly higher (P<0.05). However, PO2 of M group was significantly lower than S group (P<0.05). The above results suggested that the modeling of M group could decrease the blood oxygen pressure, while the IP group, PNU group and U group could improve the blood oxygen pressure of the rats, and the U group was superior to the IP group and the PNU group.
| Group | PO2 (mmHg) | PCO2 (mmHg) | PH |
|---|---|---|---|
| S group | 104.7 ± 9.44 | 41.6 ± 4.09 | 7.408 ± 0.036 |
| M group | 72.7 ± 9.46a | 45.3 ± 4.76 | 7.336 ± 0.036a |
| IP group | 88.3 ± 9.66b | 42.8 ± 4.57 | 7.346 ± 0.045 |
| PNU group | 88.9 ± 8.13b | 43.5 ± 3.31 | 7.343 ± 0.041 |
| U group | 97.7 ± 4.69bcd | 42 ± 4.11 | 7.35 ± 0.056 |
Table 1. The results of arterial blood gas analysis at the end of perfusion in each group.
The arterial blood pH of M group was significantly lower than that of S group, with statistical difference (P<0.05), while there was no significant difference in PCO2 between the M group and the S group (P>0.05). There was no significant difference in pH and PCO2 of U group compared with M group, IP group and PNU group, respectively (P>0.05). The pH and PCO2 of M group compared with the M group, IP group and PNU group had no statistical difference (P>0.05).
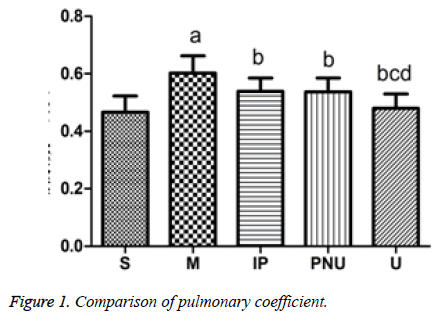
Pulmonary coefficient
Pulmonary coefficient can reflect the degree of pulmonary edema, and further indicate the level of lung injury. As illustrated in Figure 1, compared with S group, the pulmonary coefficient of M increased (aP<0.05). While the pulmonary coefficient of IP group, PNU group and U group were significantly lower than that of M group (bP<0.05). The pulmonary coefficient of U group was decreased compared with IP group and PNU group (cP<0.05, dP<0.05) as a results, the modeling of M group lead to pulmonary edema and increased pulmonary coefficient, while pulmonary edema was alleviated in IP group, PNU group and U group, U group was better than IP group and PNU group.
The HE results of lung tissue showed that the lung tissue of group S was normal. The alveolar wall of group M was extremely thickened, the alveolus was seriously collapsed, the lung tissue hemorrhage was obvious, and the interstitial inflammatory cells infiltrated; Compared with M group, Inflammatory pathological changes of IP group and PNU group were significantly reduced, the alveolar wall was moderately thickened, the alveoli was partially collapsed, the pulmonary tissue was partly hemorrhagic and there were still more inflammatory cells infiltrated in the lung group and PNU group were significantly lower than those in group M (P<0.05). There were significant relieve in inflammatory pathologic changes in U group, with mild thickening of alveolar wall, partial collapsed alveoli, partial hemorrhage of lung tissue and less infiltration of inflammatory cells.
Each group was evaluated by HE, the scores of group M was significant increased compared with S group. The scores of IP group, PNU group and U group were significantly lower than M group (bP<0.05). Compared with IP group and PNU group, the score of U group was significantly lower, with statistical difference (cP<0.05, dP<0.05). The above results demonstrated that the severe lung injury was caused by modeling in M group, IP group, PNU group and U group could alleviate lung injury, among which U group had the best effect.
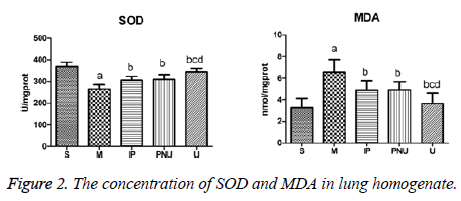
The concentration of SOD and MDA in lung homogenate
MDA is one of the representative products of lipid peroxidation, which can reflect the severity of oxidative stress, and the level in directly represents the severity of the body cells attacked by free radical. SOD is an important antioxidant in the body, indicating the level of antioxidant capacity in vivo. As shown in Figure 2, compared with S group, the concentration of MDA in M group was significantly higher than that in S group, while SOD was significantly lower (aP<0.05).
Compared with M group, the concentrations of MDA in IP group, PNU group and U group decreased and the SOD concentration increased (bP<0.05); Compared with the IP group and the PNU group, the concentration of MDA in group U was decreased and the concentration of SOD increased significantly (cP<0.05, dP<0.05). These results indicated that lipid peroxidation in lung tissue of ischemia-reperfusion model rats in M group showed a pathological increase, the anti-oxidative capacity was in pathological reduction, the IP group. The treatment of IP group, PNU group and U group could alleviate the oxidative stress in the rats lung (P<0.05), while U group was superior to IP group and PNU group. The inflammatory factors TNF-a and IL-6 can promote neutrophils, macrophages, monocytes and lymphocytes and other inflammatory cells to chemotaxis, adhesion, aggregation, activation and degranulation, as well as the release of inflammatory mediators such as lysosomal enzymes, protein hydrolase and lipid mediators, which can evaluate the extent of pulmonary inflammatory injury.
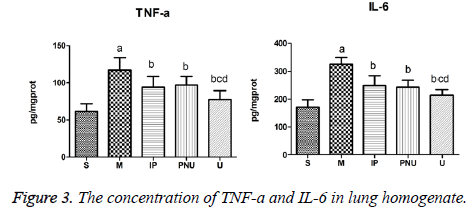
The concentration of TNF-a and IL-6 in lung homogenate
The levels of TNF-a and IL-6 in lung tissue of M group were significantly higher than those in S group (aP<0.05) Compared with M group, the levels of TNF-a and IL-6 in IP group PNU group and U group were lower (bP<0.05). TNF-a and IL-6 concentrations in U group were significantly lower than those in IP group and PNU group (cP<0.05, dP<0.05) (Figure 3). The results suggested IP group and PNU group could alleviate inflammation of lung tissue and protect the lungs, and the effect of united treatment was better.
Discussion
In this study, after 3 h-limb ischemia and 3h-reperfusion, the arterial blood PaO2 of the I/R model group decreased, indicating that the lung ventilation function was impaired, and the index of the vascular permeability-pulmonary coefficient ratio was increased. The morphology of lung tissue was observed under light microscope, the alveolar wall was extremely thickened, the alveolus was seriously collapsed, the lung tissue was hemorrhagic and the pulmonary interstitial inflammatory cells were infiltrated. The above phenomena showed that the model of lung injury induced by limb ischemia-reperfusion preparation successes.
The etiology of limb ischemia-reperfusion injury is diverse. It not only affects the survival and function of ischemic tissue, but also causes systemic inflammatory response syndrome (SIRS), leading to multiple organ dysfunction syndrome (MODS), in which the lung is one of the most vulnerable organs [22]. The clinical manifestations includes dyspnea, severe hypoxemia, acid-base balance disorders. The mechanism of LIR-ALI is complicated, including inflammatory reaction, oxidative stress, down-regulation of cystathionine-γ-lyase (CSE)/hydrogen sulfide (H2S) system, activation of renin angiotensin aldosterone system (RAAS) [23,24], in which inflammation and oxidative stress are the central links in the development of LIR-ALI.
TNF-a, as an important inflammatory mediator in the LIR-ALI process, not only produces direct cytotoxicity, but also mediates tissue damage through neutrophils (PMN), initiating a "cascade" of inflammation to increases the production of other inflammatory mediators. IL-6 is considered as an important pro-inflammatory factor to promote the activation and aggregation of PMN to aggravate lung injury. Therefore, TNF- α and IL-6 can better reflect the inflammatory process of LIRALI in rats. MDA is a kind of lipid peroxidation metabolites of polyunsaturated fatty acids, its content can reflect the degree of lipid peroxidation, and indirectly reflecting the degree of oxidative damage. SOD is the main antioxidant enzyme in vivo, and can remove oxygen free radicals to prevent cell membrane from lipid peroxidation. When a large number of free radicals are generated, the endogenous antioxidant enzyme system of the tissue is consumed in a large amount due to the participation in the anti-oxidation, and the SOD activity is decreased. As a result, MDA and SOD can reflect the oxidative stress of LIR-ALI in rats.
Ischemic preconditioning has been demonstrated to reduce ischemia-reperfusion injury in multiple tissues [25-27], and its protective effect on ischemia-reperfusion-induced lung injury has also been proved. Olguner et al. [13] found that three pretreatments of 10 min/10 min-ischemic/reperfusion can reduce lipid peroxidation level and the degree of leukocyte aggregation of lung tissue in rats treated with 4 h-lower extremity ischemia and 2 h-reperfusion. Peng et al. [14] further found that IP could reduce the injury of lung tissue induced by 3h-ischemiaand 3 h-reperfusion through inhibiting the activation of NF-κB pathway and alleviating the inflammation and oxidative stress of lung tissue mediated by HO-1. This study also demonstrated that compared with the model group, MDA, histopathological score, IL-6 and TNF-a levels of lung tissue in IP group were lower, while the level of SOD was increased, suggesting that oxidative stress and inflammation levels have improved.
Stimulation of vagal efferent nerve can cause the release of acetylcholine (Ach), Ach binds specifically to α7 nAchR on the surface of tissue macrophages to inhibit inflammatory cytokine release [28]. This Ach-mediated CAP pathway is closely related to the inflammatory response. Recent studies have shown that α7 nAchR agonists can effectively inhibit NFkB expression, cytokine release, and activation of endothelial cells and macrophage [29,30]. He et al. [31] reported that α7 nAchR agonists can reduce the levels of MDA, TNF-a and IL-6 in lung tissue and increase SOD level, thereby alleviating intestinal ischemia-induced acute lung injury. In this study, compared with the model group, in the agonist-treated group, the SOD content was increased, and the levels of SOD, MDA, IL-6, TNF-a and lung histopathological score in the lung tissue were decreased.
Compared with model group, ischemic preconditioning group and agonist-treatet group respectively, the lung pathology score, SOD, MDA, TNF-a and IL-6 in united ischemic preconditioning and α7 nAchR agonist group had statistical differences. Therefore, it is considered the united treatment group can further reduce the inflammatory response and oxidative stress in LIR-ALI rats, so as to attenuate lung injury in model rats.
I/R reperfusion results in a large number of leukocytes infiltrated, aggregation of inflammatory molecules and oxidized matter in lung tissue, and they can promote each other, further damage the integrity of pulmonary capillary endothelial cells, leading to pulmonary ventilation dysfunction [32,33]. In this study, the blood gas analysis of PO2, PCO2, pH can reflect the gas exchange function of lung. PO2 in the five groups were statistically different, the PO2 of the model group was the lowest, the PO2 of the three treatment groups was higher, and the united group was more obviously higher than the other two treatment groups. However, PCO2 and pH, similar to PO2, the difference trend displayed in each group, but they were not statistically significant. Following are possible reasons: 1) The large changes between data of the two sets of indicators and inadequate sample number; 2) The response of PCO2 and pH to lung gas exchange function was not as sensitive as PO2, but also effected by acid-base balance of the environment in vivo.
In conclusion, ischemic preconditioning and α7 nAchR agonist can reduce the limb ischemia-reperfusion lung injury in rats to some extent, and the united effect of the two is better than the single application.
Conclusion
Activation of α7 nAchR attenuates lung injury induced by limb ischemia-reperfusion in rats. Ischemic preconditioning can relieve the lung injury induced by limb ischemia-reperfusion in rats, and united treatment of ischemic preconditioning and α7 nAchR agonist can produce better lung protection. In the present study, we only studied the changes of lung injury after 3 h reperfusion in rats. We did not observe the changes of lung injury at 1 h or 2 h, and did not study the lung protection in the long-term (24 h or longer). And only four biochemical markers, such as TNF-a, IL-6, MDA and SOD, were tested, without more comprehensive testing. At last, the mechanism of elucidating the molecular level is not further elucidated in this experiment, and it needs to be further perfected and studied. The experiment is based on the basic experimental animal research. For clinical treatment, clinical practice research, such as clinical drug dose selection is in need, and the two methods of united use timing should be further explored.
References
- Korth U, Merkel G, Fernandez FF. Tourniquet-induced changes of energymetabolism in human skeletal muscle monitored by microdialysis. Anesthesiology 2000; 93: 1407-1412.
- Yeager RA, Moneta GL, Taylor LM Jr. Surgical management of severe acute lower extremity ischemia. J Vasc Surg 1992; 15: 385-391.
- Campbell WB, Collin J, Morris PJ. The mortality of abdominal aortic Aneurysm. Ann R Coll Surg Engl 1986; 68: 275-278.
- Greaves I, Porter KM. Consensus statement on crush injury and crush yndrome. Accid Emerg Nurs 2004; 12: 47-52.
- Yassin MM, Harkin DW, Barros D'Sa AA. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg 2002; 26: 115-121.
- Paterson IS, Klausner JM, Pugatch R, Allen P, Mannick JA, Shepro D, Hechtman HB. Noncardiogenic pulmonary edema after abdominal aortic aneurysm surgery. Ann Surg 1989; 209: 231-236.
- Duehrkop C, Denoyelle J, Shaw S, Rieben R. Use of dextran sulfate in tourniquet-induced skeletal muscle reperfusion injury. J Surg Res 2014; 187: 150-161.
- Kao MC, Yang CH, Chou WC. Cepharanthine mitigates lung injury in lower limb ischemia-reperfusion. J Surg Res 2015; 199: 647-656.
- Kao MC, Jan WC, Tsai PS, Wang TY, Huang CJ. Magnesium sulfate mitigates lung injury induced by bilateral lower limb ischemia-reperfusion in rats. J Surg Res 2011; 171: e97-106.
- Zhao YR, Wang D, Liu Y. The PI3K/Akt, p38MAPK, and JAK2/STAT3signaling pathways mediate the protection of SO2 against acute lung injuryinduced by limb ischemia/reperfusion in rats. J Physiol Sci 2016; 66: 229-239.
- Kao MC, Yang CH, Chou WC, Sheu JR, Huang CJ. Cepharanthine mitigates lung injury in lower limb ischemia-reperfusion. J Surg Res 2015; 199: 647-656.
- Duehrkop C, Banz Y, Spirig R. C1 esterase inhibitor reduces lower extremity ischemia/reperfusion injury and associated lung damage. PLoS One 2013; 8: e72059.
- Olguner C, Koca U, Kar A. Ischemic preconditioning attenuates the lipidperoxidation and remote lung injury in the rat model of unilateral lower limb ischemia reperfusion. Acta Anaesthesiol Scand 2006; 50: 150-155.
- Peng TC, Jan WC, Tsai PS. Heme oxygenase-1 mediates the protectiveeffects of ischemic preconditioning on mitigating lung injury induced by lower limb ischemia-reperfusion in rats. J Surg Res 2011; 167: e245-253.
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 2009; 89: 73-120.
- Wang H, Yu M, Ochani M. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421: 384-388.
- Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med 2009; 265: 663-679.
- Su X, Matthay MA, Malik AB. Requisite role of the cholinergic alpha7nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J Immunol 2010; 184: 401-410.
- Brégeon F, Xeridat F, Andreotti N. Activation of nicotinic cholinergic receptors prevents ventilator-induced lung injury in rats. PLoS One 2011; 6: e22386.
- He Y, Ye ZQ, Li X, Zhu GS, Liu Y, Yao WF, Luo GJ. Alpha7 nicotinic acetylcholine receptor activation attenuated intestine-derived acute lung injury. J Surg Res 2016; 201: 258-265.
- Mrozek JD, Smith KM, Bing DR, Meyers PA, Simonton SC, Connett JE, Mammel MC. Exogenous surfactant and partial liquid ventilation: physiologic and pathologic effects. Am J Respir Crit Care Med 1997; 156: 1058-1065.
- Punch J, Rees R, Cashmer B. Acute lung injury following reperfusion after ischemia in the hind limbs of rats. J Trauma 1991; 31: 760-765.
- Groeneveld AB, Raijmakers PG, Rauwerda JA. The inflammatory response to vascular surgery-associated ischaemia and reperfusion in man: effect on postoperative pulmonary function. Eur J Vasc Endovasc Surg 1997; 14: 351-359.
- Ning JL, Mo LW, Lu KZ, Lai XN, Wang ZG, Ma D. Lung injury following lower extremity blast trauma in rats. J Trauma Acute Care Surg 2012; 73: 1537-1544.
- Andjelkovic AV, Stamatovic SM, Keep RF. The protective effects of preconditioning on cerebral endothelial cells in vitro. J Cereb Blood Flow Metab 2003; 23: 1348-1355.
- Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg 2000; 232: 155-162.
- Aksöyek S, Cinel I, Avlan D. Intestinal ischemic preconditioning protects the intestine and reduces bacterial translocation. Shock 2002; 18: 476-480.
- de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as apharmacological target for inflammation. Br J Pharmacol 2007; 151: 915-929.
- Wang H, Liao H, Ochani M. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 2004; 10: 1216-1221.
- Saeed RW, Varma S, Peng-Nemeroff T. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med 2005; 201: 1113-1123.
- He Y, Ye ZQ, Li X, Zhu GS, Liu Y, Yao WF, Luo GJ. Alpha7 nicotinic acetylcholine receptor activation attenuated intestine-derived acute lung injury. J Surg Res 2016; 201: 258-265.
- Wang le F, Patel M, Razavi HM. Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. Am J Respir Crit Care Med 2002; 165: 1634-1639.
- Turnage RH, Guice KS, Oldham KT. Pulmonary microvascular injury following intestinal reperfusion. New Horiz 1994; 2: 463-475.