ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 4
Effect of HemoglobinA1C on the coronary flow velocity after percutaneous coronary intervention
Zheng-Bin Wang, Chung-Guang Qiu*, Shou-Jun Wang, Zhan-Ying Han, Zhen-Wen Huang, Guo-ju Sun
Department of Cardiology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
- Corresponding Author:
- Chunguang Qiu
Department of Cardiology The First Affiliated Hospital of Zhengzhou University No. 1 Construction East Road, Erqi District Zhengzhou 450052, Henan Province China
Accepted date: June 01 2014
The aims of this study was to explore the effects of hemoglobinA1C (HbA1c) levels on the flow velocity through arteries of patients treated with percutaneous coronary intervention (PCI) for acute coronary syndrome. A total of 220 acute coronary syndrome patients treated with PCI were selected and divided into two groups based on their HbA1c level on hospital admission: group A (HbA1c < 6.5%) 128 cases; group B (HbA1c ≥ 6.5%) 92 cases. The PCIrelated coronary flow velocity was confirmed using corrected TIMI frame count (CTFC). For the patients in group A, the CTFC for the left anterior descending (LAD) branch was 35.59 ± 6.84 frames, that for the left circumflex (LCX) branch was 26.55 ± 5.92 frames, and that for the right coronary artery (RCA) was 25.79 ± 6.50 frames. For the patients in group A, the CTFC levels for the LAD was 35.59 ± 6.84 frames, that for the LCX was 26.55 ± 5.92 frames, and that for the RCA was 25.79 ± 6.50 frames. The slow flow rate in group A through the LAD was 6.25%, that through the LCX was 8.1%, and that through the RCA was 8.9%; in group B, the slow flow rate through the LAD was 17.2%, that through the LCX was 25%, and that through the RCA was 26.4%. The difference was statistically significant (P < 0.05). HbA1c ≥ 6.5% significantly decreases PCI-related coronary flow velocity and increases the rate of slow flow among acute coronary syndrome patients.
Keywords
HbA1c, acute coronary syndrome, coronary flow velocity, slow-flow
Introduction
Acute coronary syndrome has a high morbidity and mortality. The 2012 ACCF/AHA Guidelines for Unstable Angina and Non-ST-segment Elevation Myocardial Infarction [1] recommends immediate percutaneous coronary intervention (PCI) for patients. The slow flow and no reflow caused by coronary intervention, which decrease perfusion to myocardial cells, may cause myocardial ischemia and significantly increase the risk of cardiovascular events [2]. This limitation greatly reduces the clinical application of PCI. HemoglobinA1C (HbA1c ) reflects the average blood glucose levels within 8 weeks to 12 weeks before testing and is the gold standard for monitoring glycemic control in diabetic patients [3]. The effect of various HbA1c levels on the flow velocity of PCI-related arteries in acute coronary syndrome patients treated with PCI has barely been reported. Hence, we explored this relationship to provide a reference for the prevention and treatment of PCI-related arterial slow flow.
Subjects and Methods
Subjects
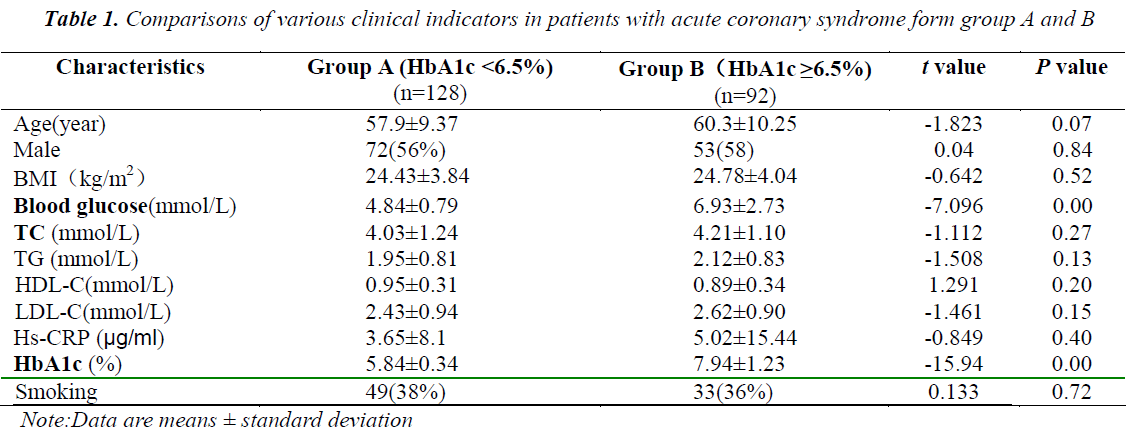
Patients hospitalized in the Department of Cardiology of the First Affiliated Hospital of Zhengzhou University from 2012 to 2013 were selected based on the inclusion criteria (diagnosis of acute coronary syndrome, coronary angiography, and PCI) and exclusion criteria (patients with old myocardial infarction, severe infections, liver disease, kidney disease, and cancer). The gender, age, smoking history, blood pressure, blood glucose, body mass index (BMI), and blood lipids of the subjects were recorded. The 220 patients were divided into the two groups based on the HbA1c at hospital admission: group A (HbA1c < 6.5%) 128 cases and group B (HbA1c ≥ 6.5%) 92 cases. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Written informed consent was obtained from all participants.
Surgery method
All patients were treated with dual antiplatelet therapy. The total preoperative dose of aspirin was greater than 300 mg and that of clopidogrel was greater than 600 mg. All patients were given oral atorvastatin and β-blockers. The patients underwent Judkins coronary angiography, and arteries with ≥75% stenosis were treated with interventional therapy. Stent types were chosen according to the lesions of the patients. Excel and Indeavor brackets were included. The stents completely covered the lesions and balloon injury segments, and the ratio of stent balloon diameter to stenosis distal diameter was 1.1:1.0. The stents were released at a pressure of 8 atm to 18 atm.
Biochemical analyses
The next morning after admission, fasting blood lipids: total cholesterol ( TC ) , Triglyceride ( TG ) , highdensity lipoprotein cholesterol(HDL-C), low-density lipoprotein cholesterol (LDL-C) and blood glucose were tested using an automatic biochemical analyzer (Cobas c 701 automatic biochemical analyzer, Germany Roche Diagnostics Ltd., Germany). HbA1c was tested using an HbA1c automatic analyzer (HLC-723G8, Tosoh Corp., Japan). High Sensitive C-Reactive Protein(hs-CRP) were tested using an automatic biochemical analyzer (Cobas c 702 automatic biochemical analyzer, Germany Roche Diagnostics Ltd., Germany).
Criteria and methods of coronary flow velocity
Corrected TIMI frame count [4] (CTFC) was used to assess the coronary flow velocity. Image acquisition was conducted at 30/s by counting the number of frames the contrast agent traveled from the coronary artery to the distal coronary artery mark. The first frame was upon injecting the contrast agent into a coronary artery and occupying the entire width of the proximal end, and the last frame was when the contrast agent reached the coronary artery distal markers. The maker at distal left anterior descending (LAD) artery was usually in the apical bifurcation. When the LAD artery covered the apex, the branches closest to the apex were selected. The marker for the right coronary artery (RCA) was the most distal bifurcation at circumflex artery and the first collateral branch of posterior descending branch at the RCA. RAO 30° + CAU 30° were observed at LAD and circumflex arteries, whereas LAO 30° + CRA 30° were observed at the RCA. Considering the LAD artery is longer than the circumflex artery and RCA, the corrected frames of the LAD artery were determined by dividing the number of frames by 1.7. The accepted normal coronary blood flow frames at the LAD artery was (36.2 ± 2.6) frames, that at the circumflex artery was (22.2 ± 4.1) frames, and that at the RCA was (20.4 ± 3.0) frames. The mean TIMI was (21.0 ± 3.1) frames. The sheets were read by two experienced interventional cardiology physicians independently and averagely. The average frames of the coronary (including the corrected frames of LAD artery) two standard deviations higher than the normal coronary flow velocity were diagnosed as coronary slow flow.
Statistical analysis
Data were analyzed through the SPSS 17.0 software. The measurement data are shown as mean ± standard deviation (±s), and the count data are expressed as percentages. The data consistent with a normal distribution were analyzed using a t-test, and the differences between groups were determined. The differences between the groups of count data were determined using aχ2 test. The significance level was at α = 0.05.
Results
Basic data
The patients in group A did not significantly differ with those in group B in terms of age, gender, BMI, blood lipids, and other indicators showed no significant difference (P > 0.05), but significantly differed in terms of HbA1c (P < 0.05) and blood glucose (Table 1).
Blood flow velocity
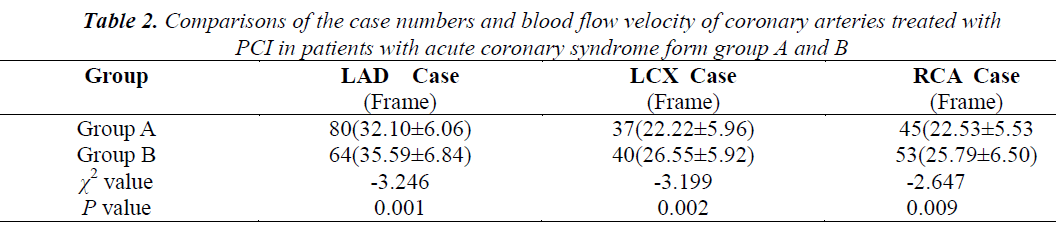
Of the patients from group A, 80 underwent PCI of the LAD and achieved a flow velocity of 32.10 ± 6.06 frames, 37 underwent PCI of the left circumflex (LCX) achieved a flow velocity of 22.22 ± 5.96 frames, and 45 underwent PCI of the RCA and achieved a flow velocity of 22.22 ± 5.96. Of the patients from group B, 64 underwent PCI of the LAD and achieved a flow velocity of 32.10 ± 6.06 frames, 40 underwent PCI of the LCX and achieved a flow velocity of 26.55 ± 5.92 frames, and 53 underwent PCI of the RCA and achieved a flow velocity of 25.79 ± 6.50 frames. The difference between the two groups was statistically significant (Table 2). Implanting coronary stents into patients with HbA1c ≥ 6.5% decreased their coronary flow velocity and reduced myocardial perfusion.
Slow-flow rate
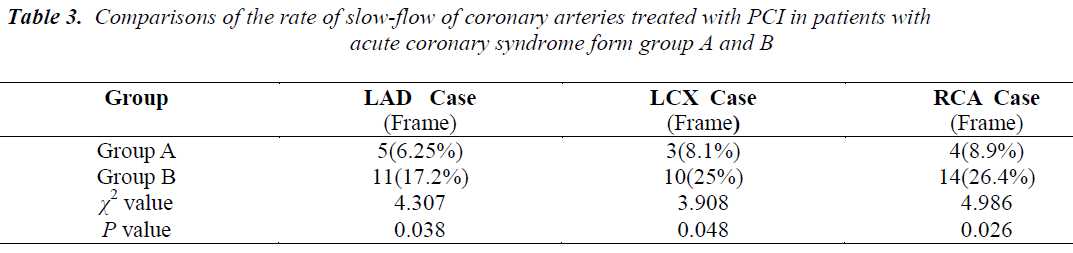
The slow flow rate of the patients from group A who underwent PCI of the LAD was 6.25%, those who underwent PCI of the LCX had 8.1%, and those who underwent PCI of the RCA had 8.9%. The slow flow rate of the patients from group B who underwent PCI of the LAD had 17.2%, those who underwent PCI of the LCX had 25%, and those who underwent PCI of the RCA had 26.4%. These results suggest that the incidence of coronary slow flow after stent implantation is significantly higher in the patients with HbA1c ≥ 6.5% that those with HbA1c < 6.5%, (Table 3).
Discussion
The extensive development of coronary intervention raised concern regarding coronary slow flow and no reflow (no-flow) among interventional physicians. The coronary slow-flow phenomenon (CSFP) refers to delayed flow of contrast agent to the distal vasculature without significant stenosis of the coronary lumen on angiography. The incidence of CSFP ranges from 5.5% to 23.7%, but its etiology and pathogenesis are unclear. CSFP may cause angina pectoris, myocardial infarction or malignant ventricular arrhythmias, and other serious cardiac adverse events [5-7], which greatly reduces the clinical usefulness of PCI for unstable angina. Therefore, the factors that affect slow flow after PCI need to be investigated to provide appropriate preventive strategies for its clinical application. Moreover, the mechanism of slow flow after PCI remains unclear; microangiopathy, endothelial dysfunction, vasomotor factor disorders, inflammation, and other phenomena are observed. These mechanisms may be related to diabetes or high levels of HbA1c.
HbA1c is hemoglobin that underwent an irreversible, non-enzymatic reaction between the free aldehyde from glucose and the valine amino of N-terminal at HbA β chain in the tissue, which is known as glycosylation. The formation of GHb depends on the blood glucose concentration and its contact time with Hb [8,9]. GHb reflects the average blood glucose levels within 8 weeks to 12 weeks prior to testing and is the gold standard for monitoring glycemic control in diabetic patients [10]. Predicting coronary heart disease events based on GHb is gaining recognition, and studies have shown that HbA1c and diabetes are closely related to cardiovascular disease [11,12]. Each 1% increase in HbA1c levels increases the relative risk of all-cause mortality by 24% (males) to 28% (females) [13]. Corpus et al. [14] reported that nondiabetic patients with coronary heart disease have significantly higher incidence of coronary, myocardial ischemic events, revascularization rate, and cardiovascular mortality and poor prognosis if their HbA1c is 6% to 7%. Increases HbA1c reflects an ongoing hyperglycemic state that injures endothelial cells. Hyperglycemia increases the release of endothelin and prostacyclin and reduces NO-based vasomotor control and glucose is directly toxic to endothelial cells and reduces their repair capacity, causing endothelial cell injury, which promotes the formation of atherosclerosis. Our study found that significantly more patients in the HbA1c ≥ 6.5% group underwent PCI of LAD, LCX, and RCA with the HbA1c < 6.5% group and they have more lesions in these three arterial branches. The CTFC frames in the HbA1c < 6.5% group were significantly higher than in the HbA1c ≥ 6.5% group, which suggest that HbA1c ≥ 6.5% after PCI slows the coronary flow velocity and increases the incidence of slow flow. HbA1c can be used as an indicator for monitoring long-term glycemic control.
HbA1c is not influenced by occasionally one-second increases and decrease and is independent of the time of blood extraction, patient fasting, insulin usage, or other unrelated factors. Compared with fasting or postprandial 2 h blood glucose, which checks immediate changes and is easily affected by eating, glucose metabolism, drug, mood, stress, and other factors, HbA1c is more objective and stable for clinical dose adjustment and treatment programs. Therefore, HbA1c has an equally important value in the controlling blood glucose levels and preventing cardiovascular events. The American Diabetes Association (ADA) classified patients with HbA1c levels of 5.7% to 6.4% as “high risk of diabetes, which suggest that patients should be advised effective strategies, such as weight loss and sports activities, to reduce the risk of cardiovascular events [15]. HbA1c directly prompts the risk of diabetes mellitus (DM) complications. A prospective study in the UK (UKPDS) demonstrated that every 1% decrease in HbA1c decreases the incidence of complications of diabetes, stroke, and myocardial infarction by 12% and 14%, decreases the incidence of cataract surgery by 19%, decreases the incidence of microangiopathy by 37%, and decreases the incidence of peripheral vascular disease that causes amputation and mortality by 43% [16]. The 2010 ADA guidelines indicated that the recommended glycemic control target in adults is HbA1c < 7%. The 2012 U.S. ACCF/AHA ST-segment elevation myocardial infarction guidelines [17] suggest that DM patients must use insulin-based programs to control blood glucose to ≤180 mg/dL during hospitalization while avoiding hypoglycemia.
TIMI flow reduction after PCI surgery should prompt examinations to rule out thrombosis, dissection, coronary spasm, or other factors that lead to cardiac coronary obstruction. Inflammation may also be involved in the pathophysiology of SCFP [18,19]. Some studies have also suggested that SCFP may be associated with hyperlipidemia, impaired glucose tolerance, smoking, BMI, and other related factors [20, 21]. In the current study, hs- CRP, smoking rates, and BMI did not significantly differ between groups A and B. Fasting blood glucose was significantly increased in the HbA1c ≥ 6.5% group, whereas PCI-related arteries flow slowed down and the slow flow incidence increased. Yılmaz et al. [22] found that HbA1c ≥ 7.0% is correlated with significantly reduced coronary flow velocity, which is consistent with the results of our study. The existence of coronary slow flow after PCI might affect the levels of myocardial perfusion and energy metabolism, thereby affecting the normal myocardial cell function and increasing cardiovascular events. Therefore, further studies on CSFP and controlling risk factors that affect coronary blood flow (e.g., abnormally elevated HbA1c) are clinically significant.
References
- 2012 Writing Committee Members, Jneid H, AndersonJL, Wright RS, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK,Zidar JP, Anderson JL. 2012 ACCF/AHA Focused Update of the Guideline for the Management of Patients with Unstable Angina/Non–ST-Elevation Myocardial Infarction (Updating the 2007 Guideline and Replacing the 2011 Focused Update). Circulation 2012; 126: 875-910.
- Cutri N, KuciaAM, Beltrame JF. Continuous ST/T wave monitoring during an acutecoronarysyndrom a presentation in patients with the C oronary slow flow phenomenon (CSFP). Heart Lung Circ (abstracts) 2008; 17: 12-20.
- Hadijadj S, DuenglerF, Barriere M. Determination of HbA1c concentrations in patients with acute myocardial infarction:comparison of the DCA 2000 device with the HPLC method. Diabetes Metab 2005; 31: 290-294.
- Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E.The TIMI 4 Study Group. TIM I frame count: a quantitative method of assessing coronary artery flow. Circulation 1996; 93: 879-888.
- Hawkins BM, Stavrakis S, Rousan TA, Abu-Fadel M, Schechter E. Coronary slow Flow- prevalence and clinical correlations. Circ J 2012; 76: 936-942.
- Goel PK, Gupta SK, Agarwal A, Kapoor A. Slow coronary flow: a distinct angiographic subgroup in syndrome X. Angiology 2001; 52: 507-514.
- Saya S, Hennebry TA, Lozano P, Lazzara R, Schechter E. Coronary slow flow phenomenon and risk for sudden cardiac death due to ventricular arrhythmias: a case report and review of literature. ClinCardiol 2008; 31: 352-355.
- American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care 2000; 23: S27-S31.
- King H, AubertRE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 1998; 21: 1414-1431.
- Hadjadj S, DuenglerF, Barriere M. Determination of HbA1c concentrations in patients with acute myocardial infarction; comparison of the DCA2000 device with the HPLC method. Diabetes Metab 2005; 31: 290-294.
- Matsushita K, Blecker S, Pazin-Filho A, Bertoni A, Chang PP, Coresh J, Selvin E. The association of hemoglobin a1c with incident heart failure among people without diabetes: the atherosclerosis risk in communities study. Diabetes 2010; 59: 2020-2026.
- Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta analysis: glycosylated hemob- globin and cardiovascular disease in diabetes mellitus. Am Intern Med 2004; 141: 421- 431.
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norflk. Ann Intern Med 2004; 141: 413-420.
- Corpus RA, O'Neill WW, Dixon SR, Timmis GC,Devlin WH. Relation of hemoglobin A1c to rate of majorasvecardiac events in non-diabetic patients undergoing percutaneous coronary revascularization. Am J Cardia 2003; 92: 1282-1286.
- American Diabetes Association. Standards of Medical Care in Diabetes--2010. Diabetes Care 2010; 33: S11-S61.
- Vlassor F. Glycatedhaemoglobin, diabetes, and mortality in men. Medicine is now using diagnostic criteria rather than reference ranges. BMJ 2001; 322: 997.
- Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, Fesmire FM, GaniatsTG, LincoffAM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American Collegeof Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am CollCardiol 2012; 60: 645-681.
- Li JJ, Qin XW, Li ZC, Zeng HS, Gao Z, Xu B, Zhang CY, Li J. Increased pdasma C-reactive protein and interleukin- 6 concentrations in patients with slow coronary flow. ClinChimActa 2007; 385: 43-47.
- Kopetz V, Penno M, Hoffmann P, Wilson DP, Beltrame JF. Investigations in the coronary slow flow phenomenon: exploring mechanisms for the acute coronary syndrome presentation. Heart Lung Circ 2008; 17S: S235.
- Binak E, Gunduz H,Sahin M, Kurtoglu N, Dindar I. The relation between impaired glucose tolerance and slow coronary flow. Int J Cardiol 2006; 111: 142-146.
- Erbay AR, TurhanH, Senen K, Yetkin O, Yasar AS, Sezgin AT, Atak R, Cehreli S, Yetkin E. Documentation of slow coronary flow by the thrombolysis in myocardial infarcion frame count in habitual smokers with angio-graphically normal coronary arteries. Heart Vessels 2004; 19: 271-274.
- Yılmaz MB, Erdem A, Yontar OC, Sarıkaya S, YılmazA, Madak N, KaradaşF, Tandoğan I. Relationship between HbA₁c and coronary flow rate in patients withtype 2 diabetes mellitus and angiographically normal coronary arteries. Turk KardiyolDernArs 2010; 38: 405-410.