ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 16
Effect of congenital and acquired HCMV infection on the expression of NMDA receptor NR1 subunit in rat offspring
Rui Zhou1, Jia-Li Xu1*, Yuan-Yuan Zhang1,3, Ting Li1,4, De Wu2 and Jiu-Lai Tang2
1Department of Paediatrics, the First Affiliated Hospital of Bengbu Medical College, Bengbu, PR China
2Department of Paediatrics, the First Affiliated Hospital of Anhui Medical University, Hefei, PR China
3The 105th Hospital of People’s Liberation Army, Hefei, PR China
4Anhui Provincial Children’s Hospital, Hefei, PR China
- *Corresponding Author:
- Jia-Li Xu
Department of Paediatrics
The First Affiliated Hospital of Bengbu Medical College
Bengbu, PR China
Accepted date: July 01, 2017
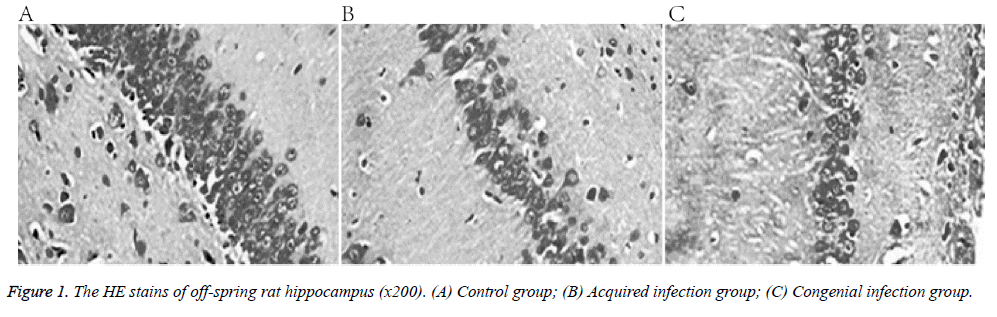
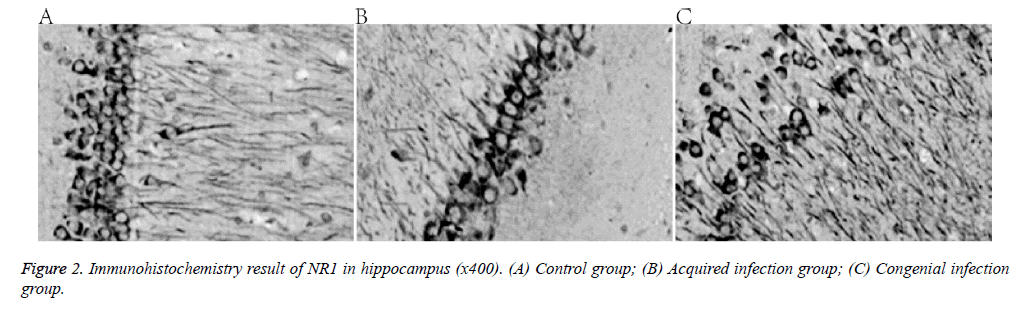
This study investigated the effect of congenital and acquired Human Cytomegalovirus (HCMV) infection on the offspring of infected rats. Eighteen 8-week old Spraque-Dawley rats were divided into three groups: control, congenital HCMV infection, and acquired HCMV infection. The offspring’s learning and memory capabilities were assessed by observing the rats in a Morris water maze. Haematoxylin-eosin staining and immunohistochemistry were applied to quantify the expression of NMethyl- D-Aspartic Acid (NMDA) receptor, net reclassification improvement (NR1) sub-unit in the hippocampus. Hippocampus structure was normal in the control group, with cells arranged evenly, intact nuclei, and obvious nucleoli. In the congenital and acquired HCMV groups, the hippocampal structure was loose, cells were reduced in granular layers, and even nuclei were lost. Furthermore, the mean absorbance of NR1, as well as learning and memory capabilities were lower in the treatment groups than in the control. Both congenital and acquired HCMV infection led to impaired learning and memory in rat offspring, possibly because of altered hippocampal expression of the NMDA receptor
Keywords
Human cytomegalovirus infection (HCMV), Learning and memory, N-methyl-D-aspartic acid receptor (NMDA), Net reclassification improvement subunit (NR1)
Introduction
Human Cytomegalovirus (HCMV) infection can be congenital, perinatal or postnatal. Congenital infection is caused mainly by intrauterine contact with the virus. Acquired infection is caused by virus-containing secretion or breast milk that is inhaled by infants when born through the obstetric canal [1]. Most studies in humans suggest that HCMV affects many organs; the most common symptoms being deafness, learning disabilities, mental retardation in children and autism [2,3].
Synaptic plasticity refers to the synapse’s ability to change shape and numbers under certain conditions. Synaptic plasticity is necessary for cognitive function and represents the neurobiological foundation of learning and memory. Considerable effort is being devoted to understanding the mechanisms responsible for impaired learning and memory [4]. Long-Term Potentiation (LTP) forms the basis of learning and memory in the central nervous system. LTP in the hippocampus has become the ideal nervous synaptic plasticity model [5]. N-methyl-D-aspartic acid (NMDA) receptor is required for signal transduction during LTP [6]. Many studies indicate that changes in expression of the NMDA receptor in the hippocampal area exert a strong influence on learning and memory, through variations in frequency of NMDA receptors and open channels [7]. The hippocampus is also the target of HCMV infection. Previous studies revealed that the hippocampus, sub-ventricular zone, and cortex were destroyed following infection with HCMV. Moreover, a prolonged infection would result in latent, discontinuously activated HCMV [2,8].
Most of the research has focused on the effect of congenital HCMV infection on learning and memory. Recently, acquired HCMV infection has gained increasing attention as it was reported that early-preterm and low-weight infants had a higher risk of HCMV-related diseases after infection through breast milk. Premature infants appeared particularly at risk of developing severe clinical symptoms, including impaired cognitive ability [9,10]. It was found that early postnatal infection with HCMV could have long-term neurobiological consequences in early-preterm infants, even as they grew older and became adolescents [11]. Most studies dealing with this topic were purely statistical and only limited basic research has been performed on congenital HCMV infection. Here, we established a convenient model for the study of congenital HCMV infection in Sprague-Dawley (SD) rats. These were treated with human embryonic fibroblasts infected with HCMV AD169, following which we detected expression of the NMDA receptor NR1 subunit in the hippocampal zone, and evaluated the animal’s learning and memory capabilities. We believe such animal models will help compare the effects of congenial and acquired HCMV infections on learning and memory.
Methods
Materials
Eight-week-old specific-pathogen-free SD rats were provided by the animal center of Anhui Medical University. They weighed 250-300 g, and included 18 females and 6 males. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Bengbu Medical University.
HCMV AD169 was provided by the Microbiology Department of Xiangya School of Medicine, CSU. Human fibroblasts were purchased from the Institute of Cell Biology (Shanghai), Chinese Academy of Sciences. High glucose DMEM was purchased from Thermo-Fisher Biochemical Products Co., Ltd. (USA).
Foetal calf serum was purchased from Hangzhou Sijiqing Biotech and trypsin was from Beyotime (both China). Primary rabbit anti-NMDA NR1 monoclonal IgG antibody was from Assay Biotech (USA) and HRP-conjugated goat anti-rabbit IgG antibody was from Beijing ZSGB-BIO. A Morris water maze was purchased from the Institute of Materia Medica (IMM), Chinese Academy of Medical Sciences. Microsyringes were from Shanghai Guangzheng Medical instrument Co., Ltd.
Model establishment
Rats were mated according to a male:female ratio of 1:3, and the vaginal plug was examined on the next morning. A total of 18 pregnant rats were randomly divided into three groups, control (n=6), congenial infection (n=6) and acquired infection (n=6). The congenital infection group rats were given 0.8 mL of a 5 × 107 L-1 HCMV suspension on the 7th day after pregnancy. In the control group, the same volume of human fibroblast’s supernatant was administered through intraperitoneal injection. The acquired HCMV group was treated with 15 μL of a 5 × 107 L-1 HCMV suspension through intracranial infection on the 2nd day postpartum.
Virus-infected human fibroblasts were allowed to proliferate according to an endpoint dilution assay. When the viral suspension showed +++~++++ within 24 h, the fibroblast’s tissue culture infectious dose 50 (TCID50) was 5 × 107 L-1.
Rats from treatment groups presented abnormal behaviour, such as listlessness, dysphoria and anorexia, after being injected with the virus. In addition, they showed hypotrichosis and dark skin, teratism, stillbirth, and stasimorphy of the embryo. Their offspring suffered from slow growth and hemiplegia. Given that control group animals manifested none of the above symptoms, we concluded that the models were successfully established.
Morris water maze test
Before the training session, rats were accustomed to the water maze for 30 s without a platform. Release positions were randomly predetermined, but the same for all rats on all trials for a given day of testing. Two types of tests were performed: (1) Located positions test.
Six 4-week-old rats were chosen randomly from each group and placed in water, once from each quadrant of the platform. The time it took rats to climb onto the underwater platform (escape latency) was recorded. The platform position was fixed. If a rat did not locate the platform within 120 s, the escape latency time was set to 120 s, the animal was placed on the platform by the experimenter and left to stay on it for 30 s.
The trial was performed 4 times per day over 4 d and the mean escape latency time was calculated. (2) Space exploration test. Once the located position test was completed, the platform was removed, the rats were placed in water, the time (within 120 s) spent in the target quadrant was measured, and the escape platform numbers were recorded.
Specimen treatment
Eight rats from each group were chosen randomly at 4 weeks postpartum and brain tissues were recovered. Specimens were fixed by soaking in 4% paraformaldehyde at 4°C for 24 h. These specimens were decalcified in 10% (w/v) ethylene diamine tetraacetic acid disodium salt solution for 10 d using a microwave rapid sample processor. After washing, the specimens were dehydrated by rising system alcohol. The specimens were soaked in xylene at room temperature for 48 h and then at 63°C for 6 h by a paraffin-embedding oven.
The specimens were embedded by a paraffin-embedding machine. Afterwards, continuous sagittal paraffin sections of 4 μm thick were sliced with a microtome. These sections were stained with a haematoxylin-eosin solution. Digital images of the stained sections were obtained and observed using a light microscope equipped with a digital camera, and image-sampling software was used to analyze the average absorbance derived from NR1 expression in the hippocampus.
Statistical analysis
Statistical analysis was performed by SPSS 16.0 software, (SPSS Inc. USA). All measurements were calculated and represented as means ± standard deviations, and Student’s t-test was used to determine statistical significant differences between the 2 groups. P<0.05 denoted a significant statistical difference.
Results
Pathological examination
The control group presented normal hippocampal structure, consisting of evenly arranged cells, with intact nuclei, obvious nucleoli, and homogeneous chromatin. In comparison, the treatment group animals showed loose hippocampal structure, with cells reduced in granular layers, and no intact nuclei (Figure 1).
Morris water maze
Contrary to the control group, both congenital and acquired infection groups showed delayed escape latency. Furthermore, results from the space exploration test indicated a statistical difference between treatment and control groups (Tables 1 and 2).
| Group | n | d1 | d2 | d3 | d4 |
|---|---|---|---|---|---|
| Normal group | 6 | 46.98 ± 16.74 | 29.83 ± 10.64 | 22.41 ± 12.16 | 15.69 ± 6.24 |
| Congenial infection group | 6 | 95.83 ± 18.36* | 70.36 ± 11.52* | 52.73 ± 11.39* | 38.96 ± 5.27* |
| Acquired infection group | 6 | 89.69 ± 16.15* | 68.27 ± 9.83* | 49.85 ± 12.21* | 35.41 ± 5.51* |
| Vs. normal group, *P<0.01. | |||||
Table 1: Comparison of escape latency among three groups in Morris water maze (s, ͞x ± s).
| Group | n | Frequency of cross platform | Swimming time/total time |
|---|---|---|---|
| Normal group | 6 | 7.95 ± 1.59 | 0.45 ± 0.12 |
| Congenial infection group | 6 | 3.96 ± 1.47* | 0.19 ± 0.16 |
| Acquired infection group | 6 | 4.38 ± 1.22* | 0.23 ± 0.10 |
| Vs. normal group, *P<0.01. | |||
Table 2: Comparison of space exploration ability among three groups in Morris water maze.
NMDA receptor NR1 subunit expression in the hippocampus
Compared with the control group, expression of the NMDA receptor NR1 subunit decreased in the two treatment groups (Figure 2 and Table 3).
| Group | n | DG | CA1 |
|---|---|---|---|
| Normal group | 8 | 0.286 ± 0.031 | 0.321 ± 0.026 |
| Congenial infection group | 8 | 0.176 ± 0.033* | 0.232 ± 0.021* |
| Acquired infection group | 8 | 0.202 ± 0.036* | 0.257 ± 0.028* |
| Vs. normal group, *P<0.01. | |||
Table 3: Absorbance value of hippocampal DG and CA1 regions among three groups.
Discussion
The NMDA receptor, a member of the glutamate receptor superfamily, is activated upon glutamate and glycine binding, resulting in the opening of a calcium ion channel. This triggers a series of cascade reactions in the postsynaptic neuron, possibly leading to the generation of NMDA receptor-dependent LTP. The NMDA receptor forms heterotetramers consisting of different ratios of essential NR1 and regionally localized NR2 subunits. NMDA receptor subunits are responsible for many features of LTP through changes in receptor numbers, subunit constitution, and distribution or self-activation of the receptor [12]. NR1 is the functional subunit of the NMDA receptor, and takes charge in the opening iron channel [13]. NR1 subunit levels in the central nervous system are initially (prenatally) low, they peak in adolescence, and then remain high throughout life both in the brain and spine. NR1 is distributed mainly in the nucleus anterior thalami, hippocampal CA1 and CA3 regions, and the Dentate Gyrus (DG) area of adult rat neurons. The latter, in particular, presents the highest expression of NR1 [14,15]. Previous studies suggest that CA1-specific knockout blocked the switch from short-term to long-term memory, leading to learning and memory dysfunction [16]. Additionally, expression of NR1 in the hippocampus has been shown to improve spatial learning and memory in a rat model of vascular dementia [17].
The Morris water maze was invented in 1984 for studying spatial learning memory in rats. It used the rodent’s innate fear of water and allowed the animals to form stable spatial cognition memories through repeated training [18]. It is employed mainly for the validation of animal neurocognitive disease models. It was found that injuries in spatial reference memory nerve regions of the hippocampus reduced the score on the Morris water maze test and led to spatial learning disorders [19,20].
HCMV belongs to the beta herpesviridae subfamily and generally induces opportunistic infections in immune compromised adults. However, it also infects growing neuronal and glial cells, causing permanent neurologic dysfunction of the developing brain. Some studies have indicated that neural stem cells and neuronal precursor cells are the targets of CMV in the developing brain, and that CMV can inhibit neuronal differentiation and induce apoptosis [21]. The NMDA receptor is the major target of HCMV [16]. Studies on primary neuronal cultures and a new born Murine Cytomegalovirus (MCMV) infection model showed that the viral antigen was found predominantly in hippocampal pyramidal neurons from the CA1 to CA3 regions. NR1 expression was decreased in the hippocampal CA1 region of MCMV-infected brains compared to that in uninfected ones, indicating that a persistent MCMV infection selectively inhibits the expression of NR1 and leads to dysfunction of the developing brain [22]. These results were similar to those reported for a congenital HCMV infection SD rat model [23].
The present study established rat models with congenital and acquired HCMV infections. These were obtained by infecting early pregnant rats and new-born rats 2 d postpartum with HCMV. Immunohistochemistry showed that the expression of NR1 was lower in hippocampal regions of both treatment groups than in the control group. The Morris water maze test showed that the learning and memory function of new-born rats was also impaired in both infection groups. Therefore, we conclude that HCMV congenital and acquired infection could impair learning and memory abilities in rats. This may be explained by the influence of HCMV on expression of the hippocampal NMDA receptors and, specifically, the NR1 subunits. Learning and memory formation are complex processes; therefore, their dysfunction is likely affected by more than one factor. The present study sheds new light on one such element; however, further research is required to understand the underlying mechanisms and develop effective prevention and treatment measures.
Conflicts of Interest
None.
Author’s Contributions
Jiali Xu and designed the study; Yuanyuan Zhang and De Wu performed the study; Jiulai Tang analyzed the data; Rui Zhou wrote the paper. All authors read and approved the final manuscript.
References
- Bale JF. Congenital cytomegalovirus infection. Handb Clin Neurol 2014; 123: 319-326.
- Nyholm JL, Schleiss MR. Prevention of maternal cytomegalovirus infection: current status and future prospects. Int J Womens Health 2010; 2: 23-35.
- Malm G, Engman ML. Congenital cytomegalovirus infections. Semin Fetal Neonatal Med 2007; 12: 154-159.
- Shonesy BC, Jalan-Sakrikar N, Cavener VS, Colbran RJ. CaMKII: a molecular substrate for synaptic plasticity and memory. Prog Mol Biol Transl Sci 2014; 122: 61-87.
- Collingridge GL, Volianskis A, Bannister N, France G, Hanna L, Mercier M, Tidball P, Fang G, Irvine MW, Costa BM, Monaghan DT, Bortolotto ZA, Molnár E, Lodge D, Jane DE. The NMDA receptor as a target for cognitive enhancement. Neuropharmacol 2013; 64: 13-26.
- Oku Y, Huganir RL. AGAP3 and Arf6 regulate trafficking of AMPA receptors and synaptic plasticity. J Neurosci 2013; 33: 12586-12598.
- Moody TD, Watabe AM, Indersmitten T, Komiyama NH, Grant SG, O’Dell TJ. β-adrenergic receptor activation rescues theta frequency stimulation-induced LTP deficits in mice expressing C-terminally truncated NMDA receptor GluN2A subunits. Learn Mem 2011; 18: 118-127.
- Pass RF. Cytomegalovirus infection. Pediatr Rev 2002; 23: 163-170.
- Lombardi G, Garofoli F, Manzoni P, Stronati M. Breast milk-acquired cytomegalovirus infection in very low birth weight infants. J Matern Fetal Neonatal Med 2012; 25 3: 57-62.
- Goelz R, Meisner C, Bevot A, Hamprecht K, Kraegeloh-Mann I, Poets CF. Long-term cognitive and neurological outcome of preterm infants with postnatally acquired CMV infection through breast milk. Arch Dis Child Fetal Neonatal Ed 2013; 98: 430-433.
- Dorn M, Lidzba K, Bevot A, Goelz R, Hauser TK, Wilke M. Long-term neurobiological consequences of early postnatal hCMV-infection in former preterms. Hum Brain Mapp 2014; 35: 2594-2606.
- Manninen T, Hituri K, Kotaleski JH, Blackwell KT, Linne ML. Postsynaptic signal transduction models for long- term potentiation and depression. Front Comput Neurosci 2010; 4: 152.
- Peng JM, Xu LS, Zhu Q, Gong S, Yu XM, Guo SY, Wu GC, Tao J, Jiang XH. Enhanced NMDA receptor NR1 phosphorylation and neuronal activity in the arcuate nucleus of hypothalamus following peripheral inflammation. Acta Pharmacol Sin 2011; 32: 160-166.
- Henson MA, Roberts AC, Salimi K, Vadlamudi S, Hamer RM, Gilmore JH, Jarskog LF, Philpot BD. Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb Cortex 2008; 18: 2560-2573.
- Burnashev N, Szepetowski P. NMDA receptor subunit mutations in neuro developmental disorders. Curr Opin Pharmacol 2015; 20: 73-82.
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Sci 2000; 290: 1170-1174.
- Zhang N, Xing M, Wang Y, Liang H, Yang Z, Shi F, Cheng Y. Hydroxysafflor yellow A improves learning and memory in a rat model of vascular dementia by increasing VEGF and NR1 in the hippocampus. Neurosci Bull 2014; 30: 417-424.
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984; 11: 47-60.
- Clausen F, Lewén A, Marklund N, Olsson Y, McArthur DL, Hillered L. Correlation of hippocampal morphological changes and Morris water maze performance after cortical contusion injury in rats. Neurosurg 2005; 57: 154-163.
- Kumaran D, Udayabanu M, Nair RU, Katyal A. Benzamide protects delayed neuronal death and behavioural impairment in a mouse model of global cerebralis chemia. Behav Brain Res 2008; 192: 178-184.
- Mutnal MB, Cheeran MC, Hu S, Lokensgard JR. Murine cytomegalovirus infection of neural stem cells alters neurogenesis in the developing brain. PLoS One 2011; 6: e16211.
- Kosugi I, Kawasaki H, Tsuchida T, Tsutsui Y. Cytomegalovirus infection inhibits the expression of N-methyl-D-aspartate receptors in the developing mouse hippocampus and primary neuronal cultures. Acta Neuropathol 2005; 109: 475-482.
- Wu D, Yang L, Xu XY, Zhang GC, Bu XS, Ruan D, Tang JL. Effects of congenital HCMV infection on synaptic plasticity indentate gyrus (DG) of rat hippocampus. Brain Res 2011; 1389: 27-34.