ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2009) Volume 20, Issue 3
Effect of Centella asiatica L. extract Against Ethinylestradiolinduced Genotoxic Damage on Cultured Human Lymphocytes
Human Genetics and Toxicology Laboratory, Section of Genetics, Department of Zoology, Faculty of Life Sciences, Aligarh Muslim University, Aligarh, India
- *Corresponding Author:
- Yasir Hasan Siddique
Human Genetics and Toxicology Laboratory
Section of Genetics, Department of Zoology
Faculty of Life Sciences, Aligarh Muslim University
Aligarh-202 002 (U.P.), India
E-mail: yasir_hasansiddique@rediffmail.com
Accepted date: March 07 2009
In this study the effect of Centella asiatica L. extract was studied against genotoxic doses of ethinylestradiol on human lymphocytes culture. The different doses of C. asiatica L. extract i.e. 1.075x10-4, 2.125x10-4 and 3.15x10-4 g/ml were treated separately with 5 μM of ethinylestradiol. Similar treatments were given with 10 μM of ethinylestradiol. The treatments result in a significant dose dependent decrease in chromosomal aberrations and sister chromatid exchanges on human lymphocyte cultures induced by 5 and 10 μM of ethinylestradiol. The selected doses of C. asiatica L. extract were not genotoxic itself. Hence it is concluded that C. asiatica L. extract reduced the genotoxic damage during the ethinylestradiol therapy in patients and thereby reducing the chances of cancer development in humans.
Keywords
Centella asiatica L, chromosomal aberrations, human lymphocytes, natural products, sister chromatid exchanges
Introduction
Centella asiatica L. belongs to the family Umbellifera. It is known as Mandukparni or Indian Penny Wort. It is found in swampy areas of India, commonly as a weed in crop fields and in other waste places throughout India up to an altitude of 600 m [1]. The crude extract of C. asiatica and the products derived from it have various pharmacological properties [2-6]. The crude extract of C. asiatica was shown to be non-toxic in normal human lymphocytes [7], and reduced the genotoxic effects of methyl methanesulphonate and cyclophosphamide in cultured human lymphocytes [8].
Conjugated estrogens, have been listed in the fourth annual report on carcinogenesis (ROC), as human carcinogens [9]. A number of individual steroidal estrogens including estradiol-17β and mestranol were listed in the ROC as reasonably anticipated human carcinogens [9]. Steroidal estrogens are placed in a Group 1 as carcinogenic to humans, on the basis of sufficient evidence of carcinogenicity in humans [10]. Ethinylestradiol is used with various progestogens, in combined oral contraceptive formulations [11]. There are sufficient evidences for the carcinogenicity of ethinylestradiol in experimental animals [12]. It has also been reported to cause aneuploidy in Chinese Hamster DON Cells in vitro [13], aneuploidy and polyploidy both, in V79 cells in vitro [14]. Estrogens are used in the treatment of sexual and metabolic disorders and also in oral contraceptives [15]. Prolonged use of estrogens has been reported to develop various types of malignancies in human and experimental animals [15]. The genotoxic effects of estrogens/ progestins can be reduced by the use of antioxidants [16-22] and natural plant products [23-28]. The genotoxicity testing provides human a risk assessment. An increase in frequency of chromosomal aberrations in peripheral blood lymphocytes is associated with an increased overall risk of cancer [29,30].
Any reduction in the genotoxicity gives an indication of the lesser possibility of carcinogenesis [31]. The majority of populations use traditionally natural preparations derived from the plant material for the treatment of various diseases, and for that reason it becomes necessary to assess the mutagenic potential or modulating action of plant extract in combination with other substances. In an earlier study, ethinylestradiol was found to be genotoxic at five and 10 μM [32]. Herbal preparations can be useful in taking care of the genotoxic effects of certain drugs. The aim of this work is to study the effect of C. asiatica L. extract against the genotoxic doses of ethinylestradiol.
Material and Methods
Chemicals
Ethinylestradiol (CAS No.: 57-63-6, Sigma); RPMI 1640, Fetal calf serum, Phytohaemagglutinin-M, Antibiotic-antimycotic mixture (Gibco); Dimethyl sulphoxide, 5-Bromo- 2-deoxy uridine; Colchicine (SRL, India), Giemsa stain (Merk).
Extract preparation
C. asiatica L. leaves were collected from the nursery of Forest Research Institute (FRI), Dehradun (UA) and were air dried and ground to fine powder. Extraction was performed by soaking samples (30 g of dry weight) in 300 ml of acetone for 8-10h at 40-60oC in Soxhlet’s apparatus. After filtration, the excess of solvent was removed by rotatory evaporator. The extract concentrations of 1.075x10-4, 2.127x10-4 and 3.15x10-4 g/ml of culture medium were established [8].
Human lymphocyte culture
Duplicate peripheral blood cultures were prepared according to Carballo et al. [33]. Briefly, heaprinized blood samples (0.5 ml), were obtained from healthy female donors and were placed in a sterile culture bottles containing seven ml of RPMI-1640 medium, supplementd with fetal calf serum (1.5 ml), antibiotic-antimycotic mixture (1.0 ml) and phytohaemagglutinin (0.1 ml). The culture bottles were kept in an incubator at 37oC for 24 hours.
Chromosomal aberration analysis
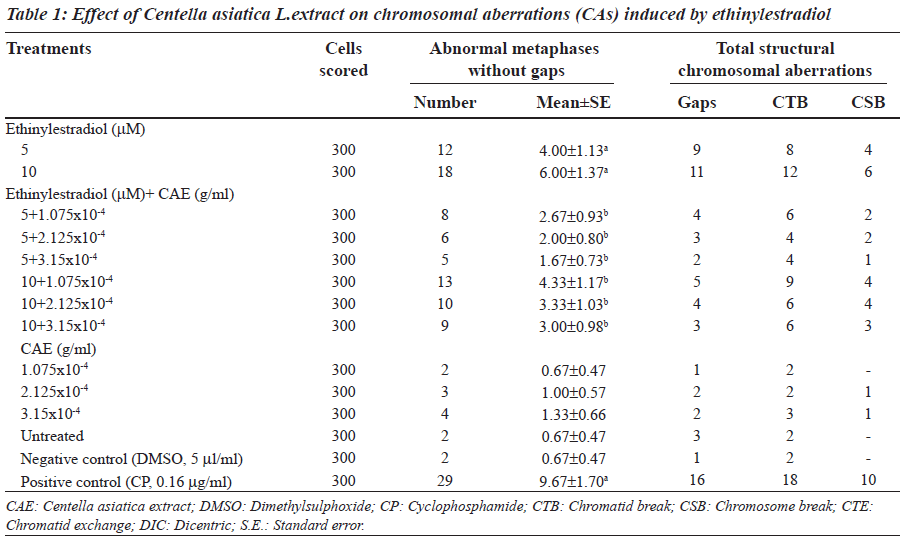
After 24 hours, 5 μM of ethinylestradiol treatment (dissolved in dimethylsulphoxide, 5 μl/ml) was given separately with 1.075x10-4, 2.125x10-4 and 3.15x10-4 g/ml of Centella asiatica L. extract. Similarly, 10 μM of ethinylestradiol was also given with the same three doses of C. asiatica extract. About, 0.5 ml of S9 mix (supplement with NADP) was also given with the treatments for six hours. The cultures were washed with fresh RPMI-1640 and kept for another 48 hours in an incubator at 37oC. After 47 hours, 0.2 ml of colchicine (0.2 μg/ml) was added to the culture bottles. Cells were centrifuged at 800 g for 10 min. The supernatant was removed and five ml of pre-warmed (37oC), KCl hypotonic solution (0.075 M) was added. Cells were resuspended and incubated at 37oC for 15 minutes. The supernatant was removed by centrifugation at 800 g for 10 minutes and five ml of chilled fixative (methanol: glacial acetic acid; 3:1) was added. The fixative was removed by centrifugation and the procedure was repeated twice. The slides were stained in three per cent Giemsa solution in phosphate buffer (pH 6.8) for 15 minutes. Three hundred metaphases were examined for the occurrence of different types of abnormally. Criteria to classify the different types of aberrations were in accordance with recommendation of EHC46 for environmental monitoring of human population [34].
Sister chromatid exchange analysis
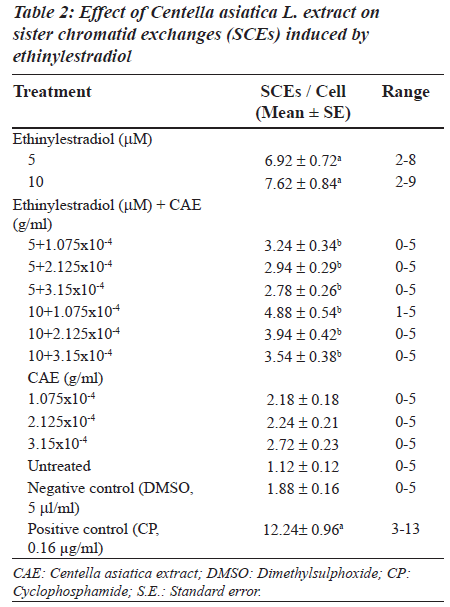
For sister chromatid exchange analysis, bromodeoxyuridine (10 μg/ml) was added at the beginning of the culture. After 24 h, 5 μM of ethinylestradiol (dissolved in dimethylsulphoxide, 5 l), the treatment was given separately with 1.075x10-4, 2.125x10-4 and 3.15x10-4 g/ml of C. asiatica extract respectively. Similar treatments with the three dosages of C. asiatica extract were given separately with 10 μM of ethinylestradiol. About, 0.5 ml of S9 mix (supplemented with NADP) was also given along with the treatments for 6 h. The cultures were washed with fresh RPMI 1640 and kept for another 48 hours, in an incubator. Mitotic arrest was performed by adding 0.2 ml of colchicine (0.2 μg/ ml). Hypotonic treatment and fixation were performed in the same way as described for the chromosomal aberration analysis. The sister chromatid exchange average was taken from an analysis of 50 metaphases during second cycle of division [35].
Statistical analysis
Student ‘t’-test was used for analysis of CAs and SCEs. Regression analysis was performed using Statistica Soft Inc.
Results
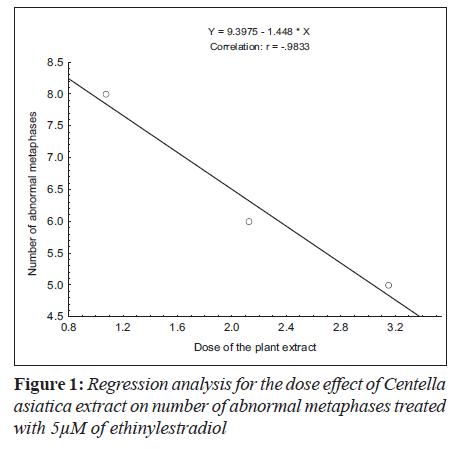
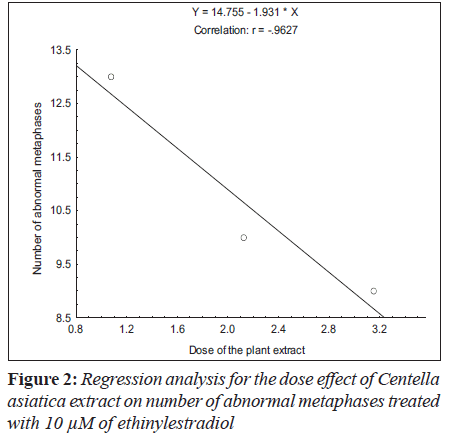
Ethinylestradiol induced a significant increase of abnormal metaphases as compared to the untreated at 5 and 10 μM. A significant dose dependent decrease in number of abnormal metaphase was observed when 5 and 10 μM of ethinylestradiol was treated separately, with the different doses of C. asiatica extract, i.e. 1.075x10-4, 2.125x10-4 and 3.15x10-4 g/ml (Table 1). For sister chromatid exchange analysis, a significant increase was observed at both of the studied doses of ethinylestradiol i.e. 5 and 10 μM (Table 2). A significant decrease in sister chromatid exchanges per cell was observed when 5 and 10 μM of ethinylestradiol was treated, separately, with the different doses of C. asiatica extract i.e. 1.075x10-4, 2.125x10-4 and 3.15x10-4 g/ml (Table 2). Regression analysis was also performed to determine the dose effect of C. asiatica extract on 5 and 10 μM of ethinylestradiol. For a number of abnormal metaphases and sister chromatid exchanges, a decrease in slope of linear regression lines with increase in dose of the extract was observed in each treatment. For abnormal metaphases the treatment of 5 μM (F = 29.14; P less than 0.04) and 10 μM (F is equal to 12.65; P < 0.05) of ethinylestradiol, with the increase in the doses of C. asiatica extract results in the decrease in the slope of the linear regression lines (Figures 1 and 2).
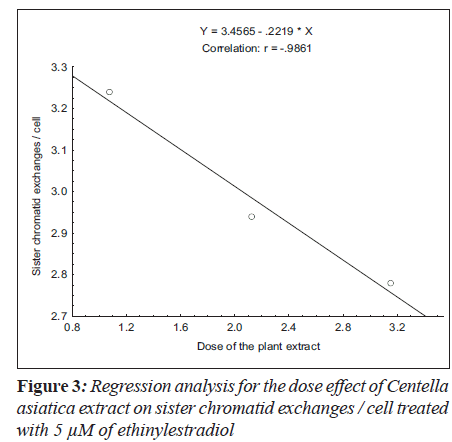
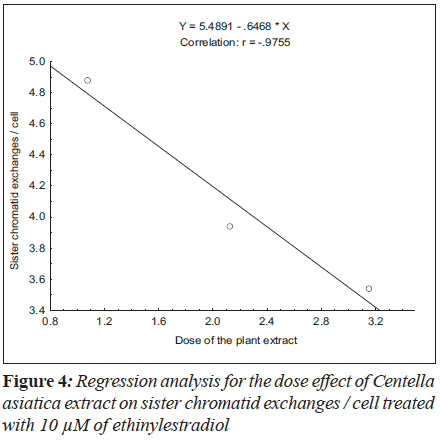
For sister chromatid exchange analysis, the treatment of 5 μM (F = 35.19; P < 0.01) and 10 μM (F = 19.69; P < 0.03) of ethinylestradiol, with the increase in the dosages of C. asiatica extract, the decrease in slope of linear regression lines was observed (Figure 3 and 4).
Discussion
The results of the study reveal that the selected dosages of the plant extract were not genotoxic per se, but reduced the genotoxic damage of ethinylestradiol on human lymphocytes in vitro. In our earlier study, three doses of ethinylestradiol (1, 5 and 10 μM) were studied [32]. Ethinylestradiol was found to be genotoxic at five and 10 μM only in the presence of S9 mix supplemented with NADP. International Agency on Cancer (IARC), mainly on the basis of epidemiological studies classifies steroidal estrogens and estrogen progestins combinations among agents carcinogenic to humans (Group 1), progestins as possibly carcinogenic (Group 2) and androgenic anabolic steroids, as probably carcinogenic (Group 2A) [36]. Cancer is characterized by genetic instability, i.e. gross chromosomal abnormalities, which consist of translocation and gene amplifications. Molecular and cytogenetic evidence indicates that the induction of structural and numerical chromosome aberrations in cell play an important role in the neoplastic development of certain tumors [37]. An increase in frequency of chromosomal aberrations in peripheral blood lymphocytes is associated with an increased overall risk of cancer [29,30]. Genetic toxicology tests are in vitro and in vivo assays designed to detect compounds that induce genetic damage directly or indirectly.
Any kind of chromosomal damage may play an important role in many malignancies. The ready quantifiable nature of sister chromatid exchanges with high sensitivity for revealing toxicant-DNA interaction and the demonstrated ability of genotoxic chemicals to induce significant increase in sister chromatid exchanges in cultured cells has resulted this endpoint being used as indicator of DNA damage in blood lymphocytes of individual exposed to genotoxic carcinogens [31]. The above genotoxic endpoints are well known markers of genotoxicity and any reduction in the frequency of these genotoxic endpoints gives us an indication of the anti-genotoxicity of a particular compound [31].
At present much attention of preventive medicine research is focused on natural antioxidants, leading to isolation and identification of new biologically active molecules by the pharmaceutical industry. The crude extract of plants may contribute to total intake of plant antioxidants and be even better source of dietary antioxidants [38].
The compounds present in the extract may inhibit the promutagen bioactivation by blocking the oxidation processes or reacts with electrophilic metabolites of promutagens [39]. The verification of the possible mutagenic and/or anti-mutagenic effects of medicinal plants infusion/ extracts is another important factor in studies. Such effects have been elucidated in some plant species by using various test systems [40]. Medicinal herbs contain complex mixtures of thousand of compounds that can exert their antioxidant and free radical scavenging effect either separately or in synergistic ways [41]. Many plant products protect against xenobiotics either by inducing defoxifying enzymes or by inhibiting oxidative enzymes [42]. Detoxification systems play a major role in preventing carcinogenesis, but also an important role in preventing xenobiotic as well as indogenous toxicity. Phytochemicals plays an important role in inhibition of carcinogenesis [43]. Some plant extract may possess substances that can modulate the genotoxicity of other compounds.
The data obtained in this study suggests that the compounds present in the extract of C. asiatica are not mutagenic on their own or when associated with ethinylestradiol in the presence of NADP.
The protective effect observed in the present study i.e. significant reduction in the frequency of cells with chromosomal damage and sister chromatid exchanges may be due to the direct action of the compounds present in the extract on ethinylestradiol by inactivating it enzymatically or chemically. The genotoxic effects of ethinylestradiol have been attributed to the bio-activation of ethinylestradiol in the presence of S9 mix supplemented with NADP [32]. The compounds present in the extract may also enhance the DNA repair system or DNA synthesis or even may prevent the bioactivation of certain chemicals [44]. Our earlier reports on synthetic progestins showed that they are genotoxic in in vitro [45-48] and in vivo [49,50]. A reduction in the frequency of SCE and chromosomal aberrations in the test system thereby indicates the possibility of reducing the chances of carcinogenesis during the ethinylestradiol therapy in patients.
Conclusion
The extract of Centella asiatica L. is potent enough to reduce the genotoxic damage of ethinylestradiol. The results of the present study suggest that the plant extract can form the basis of herbal medicine as it has antioxidants that are responsible for the reduction of the genotoxic damage of the drugs. The prolonged use of ethinyl estradiol has been reported to induce cancer. Any reduction in the frequency of CAs and SCEs thereby gives an indication of little chances of developing cancer. The extract of Centella asiatica reduces the frequency CAs and SCEs induced by ethinyl estradiol.
Acknowledgments
Thanks are due to the DST, New Delhi for awarding the project under SERC-Fast Track Scheme No: SR/FT/LS-003/2007 to the author (YHS) and to the Chairman, Department of Zoology, Aligarh Muslim University, Aligarh, India, for laboratory facilities.
References
- Dastur JF. Medicinal plants of India and Pakistan. Bombay: D.B. Taraporevala Sons and Co; 1962.
- Dutta T, Basu UP. Crude extract of Centella asiatica and products derived from its glycosides as oral antifertility agents. Indian J Exp Biol 1968;6:181-2.
- Guo JS, Cheng CL, Koo MW. Inhibitory effect s of Centella asiatica water extract and asiaticoside on inducible nitric oxide synthase during gastric ulcer healing in rats. Planta Medica 2004;70:1150-4.
- Punuree K, Wild CP, Kasinrerk W, Vinitketkumnuen U. Immunomodulatory activities of Centella asiatica and Rhimachanthus nasutus extracts. Asian Pacific J Can Prev 2005;6:396-400.
- Inamdar PK, Yeole KD, Cibogare AB, Desouza NJ. Determination of biologically active constituents in Centella asiatica. J Chromatography 1996;742:127-30.
- Yoshida M, Fuchigami M, Nago T, Okabe H, Matsunaga K, Takata J, et al. Antiproliferative constituents from Umbelliferae plants VII. Active triterpene and rosmarinic acid from Centella asiatica. Biol Pharmacol Bull 2005;28:173-5.
- Babu TD, Kuttar G, Padikkla J. Cytotoxic and antitumor properties of certain taxa of umbelliferae with special reference to Centella asiatica (L.) urban. J Ethnopharmacol 1995;48:53-7.
- Siddique YH, Ara G, Beg T, Faisal M, Ahmad M, Afzal M. Protective role of Centella asiatica L. extract against methylmethane sulphonate and cyclophosphamide induced genotoxic damage in cultured human lymphocytes. In: Phytopharmacology and Therapeutic Values I, Studium Press LLC, USA. Recent Prog Med Plants 2007;19:369- 81.
- ROC Fourth Report on Carcinogenesis, US DHHS, National Toxicology Program, 1985.
- IARC. Overall evaluations of carcinogenicity an updating of IARC monographs, Vol. 1-42. IARC Monographs on the Evaluation of Carcinogenic Risks to humans (Suppl. 7). Lyon, France: International Agency for Research on Cancer; 1987. p. 280.
- Schwend TH, Lippman JS. Comparative review of recently introduced oral contraceptives containing norgestimate, desogestrel and gestodene and older oral contraceptives. In: Paulik EJ, editor. Estrogens, Progestins and their antagonists. Boston: Birkhauser; 1996. p. 273-96.
- IARC. Sex hormones (II), IARC Monograph on the Evaluations of carcinogenic risk of chemicals to humans. Vol. 21. Lyon, France: International Agency for Research on Cancer; 1979.
- Wheeler WJ, Cherry LM, Downs T, Hsu TC. Mitotic inhibition and aneuploidy induction by naturally occurring and synthetic estrogens in Chinese Hamster Cells in vitro. Mutat Res 1986;171: 31-41.
- Sato Y, Sakakibara Y, Oda T, Aizu-Yokota E, Ichinoseki K. Effect of estradiol and ethinylestradiol on microtubule distribution in Chinese Hamster V79 Cells. Chem Pharmcol Bull 1992;40:182-4.
- IARC. Post menopausal oestrogen therapy. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 72. Lyon, France: International Agency for Research on Cancer; 1999. p. 399.
- Siddique YH, Ara G, Beg T, Afzal M. Additive action of vitamin C and E against norgestrel induced genotoxicity. Biomed Res 2007;18:155-60.
- Siddique YH, Beg T, Afzal M. Antigenotoxic effects of ascorbic acid against megestrol acetate induced genotoxicity in mice. Hum Exp Toxicol 2005;24:121-7.
- Siddique YH, Ara G, Beg T, Afzal M. Effect of vitamin C on cyproterone acetate induced genotoxic damage in mice. Res J Biol Sci 2006;1:69-73.
- Siddique YH, Beg T, Afzal M. Anticlastogenic effects of ascorbic acid against the genotoxic damage induced by norethynodrel. Adv Environ Biol 2007;1:27-32.
- Siddique YH, Afzal M. Protective role of allicin and L. ascorbic acid against the genotoxic damage induced by chlormadinone acetate in cultured human lymphocytes. Indian J Exp Biol 2005;49:769-72.
- Siddique YH, Beg T, Ara G, Gupta J, Afzal M. Antigenotoxic effect of allicin against estradiol-17β induced genotoxic damage in cultured mammalian cells. Natural Products Research (in press)
- Siddique YH, Beg T, Afzal M. Antigenotoxic effect of ascorbic acid against cypropterone acetate induced genotoxicity in cultured mammalian cells. In: Siddique YH, editor. Recent Trends in Toxicology. Trivandpuram, Kerala, India: Transworld Research Network; 2008. p. 85-94.
- Siddique YH, Ara G, Beg T, Afzal M. Antigenotoxic effect of Ocimum sanctum L. extract against cyproterone acetate induced genotoxic damage in cultured mammalian cells. Acta Biol Hungarica 2007;58:397-407.
- Siddique YH, Ara G, Beg T, Afzal M. Antigenotoxic effect of nordihydroguaiaretic acid (NDGA) against chlormadinone acetate induced genotoxic damage in mice bone marrow cells. J Nat Med 2008;62:52-6.
- Siddique YH, Beg T, Afzal M. Protective effect of nordihydroguaiaretic acid (NADGA) against norgestrel induced genotoxic damage. Toxicol in vitro 2006;20:227- 33.
- Siddique YH, Ara G, Beg T, Faisal M, Ahmad M, Afzal M. Antigenotoxic role of Centella asiatica L. extract against cyproterone acetate induced genotoxic damage in cultured human lymphocytes. Toxicol Invitro 2008;22:10-7.
- Siddique YH, Ara G, Beg T, Afzal M. Possible modulating action of plant infusion of Ocimum sanctum L. on chromosomal aberrations and sister chromatid exchanges induced by chlormadinone acetate in human lymphocytes invitro. J Environ Biol 2008;29:845-8.
- Siddique YH, Ara G, Beg T, Afzal M. Protective role of nordihydroguairetic acid (NDGA) against the genotoxic damage induced by ethinodiol diacetate in human lymphocytes invitro. J Environ Biol 2007;28:279-82.
- Hagmar L, Bonassi S, Stromberg U, Brogger A, Knudson JE, Norppa H, et al. Chromosomal aberrations in human lymphocytes predicts human cancer: A report from the European study group on cytogenetic biomarkersnd health (ESCH). Cancer Res 1998;58:4117-21.
- Hagmar L, Brogger A, Hansteen IL, Heim S, Hogstedt B, Knudsen L, et al. Cancer risk in humans predicted by increased level of chromosomal aberration in human lymphocytes. Nordic study group on the health risk of chromosome damage. Cancer Res 1994;54:2919-22.
- Albertini RJ, Anderson D, Douglas GR, Hagmar L, Heminki K, Merelo F, et al. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. Mutat Res 2000;463:119-72.
- Siddique YH, Beg T, Afzal M. Genotoxic potential of ethinylestradiol in cultured mammalian cells. Chem Biol Interact 2005;151:133-41.
- Carballo MA, Alvarez S, Boveris S. Cellular stress by light and Rose Bengal in human lymphocytes. Mutat Res 1993;288:215-22.
- IPCS. Internatinal Programme on Chemical Safety: Environment Health Criteria 46. Guidelines for the study of genetic effects in human populations. Geneva: WHO; 1985. p. 25-54.
- Perry P, Wolff S. New Giemsa methods for differential staining of sister chromatids. Nature 1974;261:156-8.
- Martelli A, Mattioli F, Angiola M, Reimann R, Brambilla G. Species, sex and inter-individual differences in DNA repair induced by nine sex steroids in primary culture of rat and human hepatocytes. Mutat Res 2003;536:69-78.
- Yunis JJ. The chromosomal basis of human neoplasia. Science 1983;221:227-36.
- Zamorano-Ponce E, Morales C, Ramos D, Sepulveda C, Cares S, Rivera P, et al. Antigenotoxic effect of Aloysia triphylla infusion against acrylamide induced DNA damage as shown by the comet assay technique. Mutat Res 2006;603:145-50.
- Kaya B. Antigenotoxic effect of ascorbic acid on mutagenic dose of three alkylating agents. Turkish J Biol 2003;27:241-6.
- Roncada T, Vicentini VE, Mantovani MS. Possible modulating actions of plant extracts on the chromosome breaking activity of MMC and Ara C in human lymphocytes in vitro. Toxicol in vitro 2004;18:617-22.
- Romero-Jimenez M, Sanchez JC, Analla M, Serrano AM, Moraga AA. Genotoxicity and antigenotoxicity of some traditional medicinal herbs. Mutat Res 2005;585:147-55.
- Morse MA, Stoner GD. Cancer chemoprevention: principles and prospects. Carcinogenesis 1993;114:1737- 46.
- Blaylock RL. A review of conventional cancer prevention and treatment and the adjunctive use of nutraceutical supplements and antioxidants: Is there a danger or a significant benefit? J Am Nutrac Assoc 2000;3:75-95.
- Kuroda Y, Jain AK, Tezuka H, Kada T. Antimutagenicity in cultured mammalian cells. Mutat Res 1992;267:201-9.
- Siddique YH, Ara G, Beg T, Afzal M. Genotoxic potential of medroxyprogesterone acetate in cultured human peripheral blood lymphocytes. Life Sci 2006;80:212-8.
- Siddique YH, Afzal M. Genotoxic potential of cyproterone acetate of possible role of reactive oxygen species. Toxicol In vitro 2005;19:63-8.
- Siddique YH, Afzal M. Evaluation of genotoxic potential of norethynondrel in human lymphocytes in vitro. J Environ Biol 2005;26:387-92.
- Siddique YH, Afzal M. Evaluation of genotoxic potential of ethynodiol diacetate in human lymphocytes in vitro. Curr Sci 2004;86:1161-5.
- Siddique YH, Afzal M. Evaluation of genotoxic potential of synthetic progestin chlormadinone acetate. Toxicol Lett 2004;15:221-5.
- Siddique YH, Afzal M. in vivo evaluation of sister chromatid exchanges (SCEs) and chromosomal aberrations (CAs) by lynestrenol. Indian J Exp Boil 2005;43:291-3.