ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2014) Volume 25, Issue 3
Effect and mechanism of action of cinnamic acid on the proliferation and apoptosis of leukaemia cells.
Department of Pediatrics, The First Affiliated Hospital of Xinxiang Medical University, Weihui 453100, China
- *Corresponding Author:
- Taixin Shi
Department of Pediatrics
The First Affiliated Hospital of Xinxiang
Medical University
No. 88 Jiankang Road, Weihui 453100,China
Accepted May 14 2014
This study aims to investigate the effect and mechanism of action of cinnamic acid (CA) on the proliferation and apoptosis of leukaemia K562 cells. Morphological changes in cells were observed in K562 cells treated by CA with different doses (1.5, 3.0, and 6.0 mg/mL). Cell proliferation capabilities were measured by methyl thiazolyl tetrazolium assay. Cell cycle was determined by flow cytometry, and the rate of cell colony formation was calculated by colony test. CA treatment for 24, 48, 72, and 96 h significantly inhibited the growth of leukaemia cells. CA significantly affected the cell cycle after 48 h treatment, resulting in increased G0/G1 phase and extension of the cell cycle in the tumour cells. Moreover, the colony formation rate decreased significantly (P < 0. 05). CA can inhibit the growth of leukaemia cells by inducing cell apoptosis.
Keywords
Leukaemia, Cinnamic acid, proliferation, apoptosis.
Introduction
Food and environmental factors play an important role in the occurrence and development of tumours [1-4]. However, treatment of cancer by traditional agents has adverse side effects, thereby inducing tumour cells to differentiate into normal cells. Efficient agents with low toxicity are important in cancer treatment. Cinnamic acid (CA), a phenylalanine deamination product in plant tissue, is the main active ingredient extracted from food-flavouring agents. CA is a hormone-like substance, which regulates cell growth and differentiation in plants. CA is a safe and extensive source material because of its natural and low toxicity properties [5,6]. Metabolites, such as benzoic and hippuric acid, in the human body have no proliferation inhibition and cytotoxicity in normal or tumour cells [7]. Therefore, changing eating habits by avoiding carcinogenic food and promoting the use of food with cancer-preventing and anti-cancer effects are important. CA may be an effective food-flavouring agent with cancer-preventing and anticancer effects [8,9]. In this study, different doses of CA were used to treat leukaemia K562 cells. The effect and mechanism of action of CA on the proliferation and apoptosis of leukaemia cells were observed.
Materials and Methods
Cinnamic acid
Cinnamic acid (CAS number 140-10-3) was obtained as trans-cinnamic acid crystals, 99 + % (Sigma Aldrich Chemical Company Inc.) and the solutions were prepared by using 24 mg of the compound and 500 μL of ethanol. Phosphate buffered saline (PBSA) was added to complete 10 mL (final concentration at 16 mM). An appropriate control with DMEM, 20% PBSA and 1% ethanol was used.
Cell culture and grouping
Human leukaemia K562 cell lines stored in our laboratory were cultured in PRMI-1640 medium containing 10% fetal bovine serum, 100 U/mL penicillin, and l00 U/mL streptomycin in a humidified incubator at 37 °C and 5% CO2, passaged once every 2 d to 3 d. Logarithmic growth phase passaged cells were seeded in 24-well culture plate, and three doses of CA were added to the culture medium to final concentrations of 1.5, 3.0, and 6.0 mg/mL. The medium was changed every other day, and specimens were collected every day. Cell culture medium without drug treatment was designated as the blank control group.
Cell morphology
After Giemsa staining, morphological changes of the cells treated with CA in concentrations of 1.5, 3.0, and 6.0 mg/mL (dissolved in a final volume fraction <0.1% of DMSO) for 24, 48, 72, and 96 h were observed under an inverted microscope.
MTT assay
The drug-treated group was treated with CA in final concentrations of 1.5, 3.0, and 6.0 mg/mL, whereas no drugs were used for the control group. The solvent control group was treated with culture medium containing DMSO with equal concentrations, and the culture medium without cells was assigned as a blank control. After culturing for 24, 48, 72, and 96 h, the absorbance (A) of each well was measured under a microplate with a wavelength of 492 nm, and the cell growth inhibition rate (%) was calculated as follows: Cell growth inhibition rate (%) = (control A-test A)/(control A-blank A)×100.
Flow cytometric analysis
CA with final concentrations of 1.5, 3.0, and 6.0 mg/mL was added to leukaemia cells with well growth, and the cells were incubated for 48 h. The cells were then collected, determined by flow cytometry, and analysed to obtain the percentage (%) for the cell cycle (G0 / G1, S, G2 / M) at each time point.
Plate cloning experiments
The drug-treated groups were cultured in a medium containing CA with final concentrations of 1.5, 3.0, and 6.0 mg/mL for 24 h and added with RPMI01640 medium containing the new fetal bovine serum with a volume fraction of 10% to further culture for 18 d. After immersing, fixing, and staining, the clones (clone with more than 50 cells was considered to be a clone) in each group were counted. Colony formation and inhibition rates were calculated as follows: Clone formation rate (%) = average number of clones/number of seeded cells×100; and Inhibition rate (%) = (1-clones number of the experimental group/clones number of the control group) × 100.
Statistical analysis
Data were expressed as x ±s. SPSS13.0 statistical software package was used to perform univariate ANOVA. Pairwise comparisons were performed by t-test.
Results
Morphological changes
With increased dose and prolonged drug treatment time, the cells in the treated group showed the following characteristics: the stacking nature of cells during growth disappeared, cells proliferated slowly, they shrank, cytoplasmic granules increased, and some cells became round and bloated. Cell growth in the control group was adherent with epithelioid type, with two to three processes and homogeneous and transparent in nature (with two to four nucleoli), clear nuclear membrane and nucleolus outlines, and compact structures between cells. The cells grew progressively.
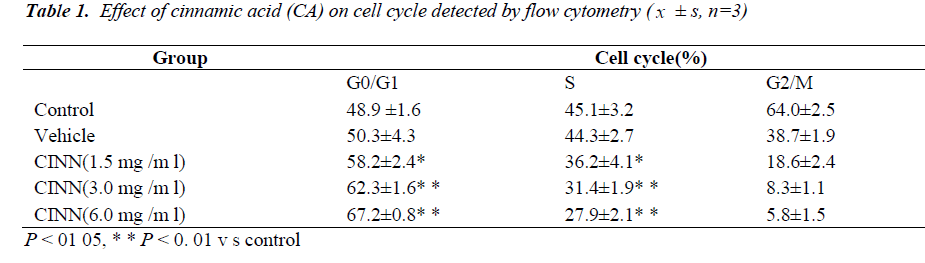
Growth inhibitory effect
CA in concentrations of 1.5, 3.0, and 6.0 mg/mL inhibited the growth of leukaemia cells, and the inhibition gradually increased with prolonged treatment and increased concentration (Figure 1).
Cell cycle
As shown in Table 1, after increasing the dose of CA (1.5, 3.0, and 6.0 mg/mL) treatment to 48 h, cell proportion of G0/G1 phase increased (P < 0.01), whereas cell proportion of S phase decreased (P < 0.05).
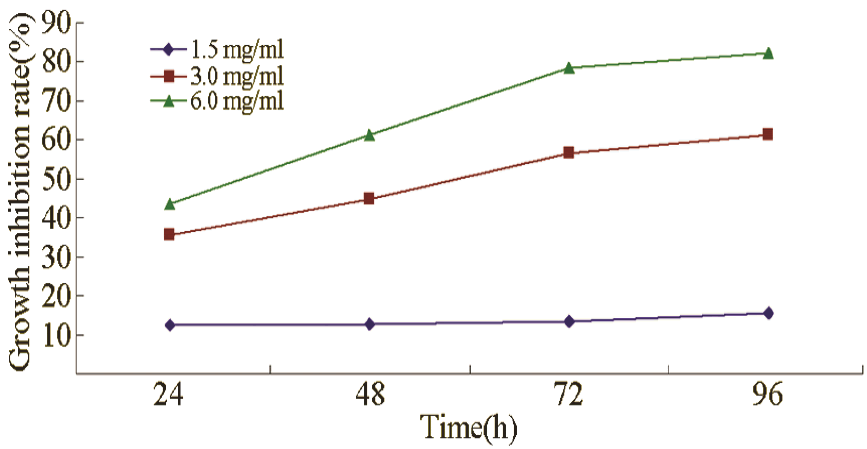
Cell viability
As shown in Table 2, after CA treatment (1.5, 3.0, and 6.0 mg/mL), the number of colony formation for leukaemia cells was reduced, and the colony formation rate was significantly lower than that of the control group (P < 0.05).
Discussion
CA is a chemical occurring naturally in plants. This molecule belongs to aromatic fatty acids and is a phenylalanine deamination product in plant tissues. Few studies have reported on the effects of CA on tumour cells. Tsai et al. explored the mechanisms underlying several select cinnamic acid derivatives against invasion of human lung adenocarcinoma A549 cells, and found that caffeic acid, chlorogenic acid, and ferulic acid can inhibit phorbol-12- myristate-13-acetate-stimulated invasion of A549 cells at a concentration of ≥100 μM. The MMP-9 activity was suppressed by these compounds through regulating urokinase-type plasminogen activator, tissue inhibitor of metalloproteinase -1, plasminogen activator inhibitor -1, and PAI-2; the cell-matrix adhesion was decreased by CAA only. The proposed molecular mechanism involved not only decreasing the signaling of MAPK and PI3K/Akt but also inactivating NF-κB, AP-1, and STAT3 [10]. Sova et al. investigated the cytotoxic effects of representative cinnamic acid esters and amides, and found that the compounds showed significant cytotoxicity (IC50s between 42 and 166 μM) and selectivity of these cytotoxic effects on the malignant cell lines. The cell cycle phase distribution indicated that novel cinnamic acid derivatives inhibited cell growth by induction of cell death [11].
CA with different concentrations was used to treat leukaemia cell line K562. CA with concentrations of 1.5, 3.0, and 6.0 mg/mL inhibited the growth and proliferation of K562 cells in time-and dose-dependent manners. In addition, morphological observation showed that the stack growth state of K562 cells disappeared after CA treatment, suggesting that the contact inhibition of the cell growth may be restored. Cell proliferation was negatively correlated with differentiation [12-14]. The slowing speed of cell division and proliferation is often the result of cell differentiation.
The G1 phase is considered a cell differentiation phase. The arrest of the G1 phase for the cell cycle can be used as indicator of induced differentiation [15,16]. The experimental results showed that the K562 cell cycle was arrested in the G0/G1 phase after CA treatment. The effect obviously increased with increased dose, indicating that CA induces differentiation of cells in a dosedependent manner.
The exact mechanism of the antitumor effect of CA is still unclear. Two possible mechanisms were considered [17]: 1) CA activates the human peroxisome proliferator and nuclear receptor to inhibit oncogene (may be c-myc) amplification and expression of other genes; 2) CA blocks the posttranslational modification of cell growth-regulating proteins, such as certain p21ras protein, that is, preventing protein prenylation by inhibiting the synthesis of mevalonatederived residues. These residues cannot bind strongly to the cell membrane, thereby inhibiting cell growth.
CA induced the differentiation of the cultured leukaemia cells. Whether this same effect existed in vivo will be further analysed by our study team. Actively exploring the mechanism of CA induction of tumour cell differentiation will help achieve an effective cancer-preventing and anti-cancer therapy, reduce or avoid the pain in the cancer patients caused by the toxic effects of traditional treatments, and facilitate new cancer treatment pathways.
References
- Santhosh PB, Ulrih NP. Multifunctional superpara- magnetic iron oxide nanoparticles: promising tools in cancer theranostics. Cancer Lett 2013; 336: 8-17.
- Salehi M, Moradi-Lakeh M, Salehi MH, et al. Meat,fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutr Rev 2013; 71: 257-267.
- Nielsen GD, Larsen ST, Wolkoff P. Recent trend inrisk assessment of formaldehyde exposures from in- door air. Arch Toxicol 2013; 87: 73-98.
- Simate GS, Iyuke SE, Ndlovu S, Heydenrych M,Walubita LF. Human health effects of residual car- bon nanotubes and traditional water treatment chemicals in drinking water. Environ Int 2012; 39: 38-49.
- RIFM Expert Panel, Belsito D, Bickers D, Bruze M, Calow P, Dagli ML, Dekant W, Fryer AD, Greim H, Miyachi Y, Saurat JH, Sipes IG. A toxicologic and dermatologic assessment of cinnamyl phenylpropyl materials when used as fragrance ingredients. Food Chem Toxicol 2011; 49: S256-267.
- Adisakwattana S, Sompong W, Meeprom A, Nga- mukote S, Yibchok-Anun S. Cinnamic Acid and its derivatives inhibit fructose-mediated protein glyca- tion. Int J Mol Sci 2012; 13: 1778-1789.
- Liu L, Hudgins WR, Shack S, Yin MQ, Samid D. Cinnamic acid: a natural product with potential use in cancer intervention. In t J Cancer 1995; 62: 345- 350.
- Niero EL, Machado-Santelli GM. Cinnamic acid induces apoptotic cell death and cytoskeleton disrup- tion in human melanoma cells. J Exp Clin Cancer Res 2013; 32: 31.
- Yao K, Youn H, Gao X, Huang B, Zhou F, Li B, HanH. Casein kinase 2 inhibition attenuates androgen receptor function and cell proliferation in prostate cancer cells. Prostate 2012; 72: 1423-1430.
- Tsai CM, Yen GC, Sun FM, Yang SF, Weng CJ. As-sessment of the anti-invasion potential and mecha- nism of select cinnamic acid derivatives on human lung adenocarcinoma cells. Mol Pharm 2013; 10: 1890-1900.
- Sova M, Žižak Ž, Stanković JA, Prijatelj M, Turk S, Juranić ZD, Mlinarič-Raščan I, Gobec S. Cinnamic acid derivatives induce cell cycle arrest in carcinoma cell lines. Med Chem 2013; 9: 633-641.
- Zou CP, Clifford JL, Xu XC, Sacks PG, Chambon P, Hong WK, Lotan R. Modulation by retinoic acid (RA) of squamous cell differentiation, cellular RA- binding proteins, and nuclear RA receptors in human head and neck squamous cell carcinoma cell lines. Cancer Res 1994; 54: 5479-5487.
- An Y, Kang Q, Zhao Y, Hu X, Li N. Lats2 Modu-lates Adipocyte Proliferation and Differentiation via Hippo Signaling. PLoS One 2013; 8: e72042.
- Liu Y, Lv L, Xue Q, Wan C, Ni T, Chen B, Liu Y, Zhou Y, Ni R, Mao G. Vacuolar protein sorting 4B, an ATPase protein positively regulates the progres- sion of NSCLC via promoting cell division. Mol Cell Biochem 2013; 381: 163-171.
- Wang B, Zhang Y, Zhang X. Attenuation of krüppel- like factor 4 facilitates carcinogenesis by inducing g1/s phase arrest in clear cell renal cell carcinoma. PLoS One 2013; 8: e67758.
- Rizzardi LF, Cook JG. Flipping the switch from g1 to s phase with e3 ubiquitin ligases. Genes Cancer 2012; 3: 634-648.
- Goldstein JL, BrownM. Regulation of the mevalo- nate pathway. Nature 1990; 343: 425-430.
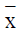 ± s, n=3)
± s, n=3)