ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 5
Direct repair of defects in lumbar spondylolysis by using a combination of computer-assisted minimally invasive spine surgery and the Buck technique
The Buck technique has been used for a long time in treating lumbar spondylolysis; however, the use of this technique was limited by defects such as screw misplacement. Nowadays, the Computer-Assisted Minimally Invasive Spine Surgery (CAMISS) technique is widely used in spine surgery; however, research on the treatment of lumbar spondylolysis has rarely been reported. In our study, 23 patients with lumbar spondylolysis were managed with CAMISS-Buck surgery. The average operative duration was 97.8 ± 14.9 min, and amount of blood loss was 38.0 ± 15.6 ml. Among all 23 patients, 22 were followed for 24.2 months on an average. The postoperative and follow-up Oswestry Disability Index and the postoperative and follow-up visual analogue scale scores significantly improved compared with the preoperative values. According to the follow-up radiographic results, the screw implantation was excellent (rate, 100%) and the solid fusion rate was 81.8%. In addition, no postoperative complications were observed. In conclusion, the CAMISS-Buck technique is a feasible approach in treating lumbar spondylolysis.
Keywords
Buck’s technique, Computer-assisted minimally invasive spine surgery (CAMISS), Spondylolysis.
Introduction
Lumbar spondylolysis is a common cause of lower back pain [1]. The incidence is about 3-6% among young people, and it is unlikely to heal by itself [2]. Most researches have proven that spondylolysis is caused by fatigue fracture of the isthmus [3-6]. Most patients with lumbar spondylolysis are asymptomatic. For patients with symptoms, conservative treatment is the first choice. Most symptoms can be relieved with bed rest, bracing protection, and using non-steroidal drugs [1,7-9]. However, for some patients, the symptoms persist or even become aggravated after a long time of conservative treatment. In these cases, surgery should be considered [10].
Buck was the first to propose the concept of direct isthmus fixation [11]. Since the 1970’s, many surgeons have used this technique [12-15] to treat lumbar spondylolysis and have achieved good results. However, owing to dysplasia and the small size of isthmus, the conventional methods of screw implantation can easily lead to misplacement, screw loosening, screw breakage, or even irreversible damage to the nerve root during the surgery [16].
With the help of an intraoperative computer-assisted navigation system, surgeons can precisely locate the orbit of a fixation device, which can greatly enhance the accuracy of the fixation implantation [17]. Our department has employed this technique in the upper cervical [18,19] and lumbar vertebrae with axial rotation [20] previously, and demonstrated the safety and accuracy of pedicle screw placement. On this basis, the Computer-Assisted Minimally Invasive Spine Surgery (CAMISS) technique combines the advantages of navigation technology and minimal invasiveness, making surgery less injurious and more accurate, thereby accelerating postoperative recovery. We used the CAMISS method in treating degenerative lumbar disease and achieved a good outcome [21].
In 2013, our department began to use the CAMISS technique to treat lumbar spondylolysis. With this technique, surgeons can accurately place the isthmus screws and overcome the difficulties in screw implantation with the traditional Buck method.
Materials and Methods
Patients
Twenty-three patients with a diagnosis of lumbar spondylolysis were enrolled in our retrospective study from January 2013 to December 2015. All of them accepted the CAMISS-Buck surgery. Among the 23 patients, 18 were male and 5 were female (male-to-female ratio, 3.6:1). The average age was 22.9 years (range, 14-35 years). Among them, two patients were older than 30 years. Concerning their occupation, 5 were students, 14 were manual workers, 2 were athletes, and 2 patients were office workers. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Tsinghua University. Written informed consent was obtained from all participants.
Low back pain was the chief complaint for all patients, including three with accompanying hip pain. The symptom duration was 4.6 years on average. All patients had failed results after at least 6 months of conservative treatment. Preoperatively, all patients took the vertebral isthmus closure test to confirm the relationship between low back pain and lumbar spondylolysis [22].
All patients took lumbar static and dynamic radiographs, Computed Tomography (CT), and Magnetic Resonance Imaging (MRI) to confirm the presence of lumbar spondylolysis. According to the disc signal indicated on the lumbar MRI, all patients were classified as class I (Pfirrmann grading method) [23] without spondylolisthesis, lumbar disc herniation, spinal stenosis, and other lumbar degenerative diseases. All the 23 patients had single-segment spondylolysis with both sides involved, at L4 in 6 cases and L5 in 17 cases (Table 1).
| Case | Gender | Age | Occupation | Spondylolysis Segment | Spondylolysis Position | Case | Gender | Age | Occupation | Spondylolysis Segment | Spondylolysis Position |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 29 | Manual worker | L5 | Bilateral | 14 | Female | 20 | Manual worker | L5 | Bilateral |
| 2 | Male | 27 | Office staff | L5 | Bilateral | 15 | Female | 21 | Manual worker | L5 | Bilateral |
| 3 | Male | 14 | Student | L5 | Bilateral | 16 | Male | 15 | Student | L4 | Bilateral |
| 4 | Male | 29 | Manual worker | L4 | Bilateral | 17 | Male | 17 | athletes | L5 | Bilateral |
| 5 | Male | 27 | Manual worker | L5 | Bilateral | 18 | Male | 19 | Manual worker | L4 | Bilateral |
| 6 | Male | 35 | Manual worker | L5 | Bilateral | 19 | Male | 22 | Manual worker | L5 | Bilateral |
| 7 | Male | 25 | Manual worker | L5 | Bilateral | 20 | Female | 27 | Office staff | L4 | Bilateral |
| 8 | Male | 22 | Student | L4 | Bilateral | 21 | Male | 18 | athletes | L5 | Bilateral |
| 9 | Male | 21 | Student | L4 | Bilateral | 22 | Male | 16 | Student | L5 | Bilateral |
| 10 | Female | 33 | Manual worker | L5 | Bilateral | 23 | Male | 20 | Manual worker | L5 | Bilateral |
| 11 | Male | 18 | Manual worker | L5 | Bilateral | - | - | - | - | - | - |
| 12 | Male | 22 | Manual worker | L5 | Bilateral | - | - | - | - | - | - |
| 13 | Male | 29 | Manual worker | L5 | Bilateral | - | - | - | - | - | - |
Table 1. Detailed information of all the fourteen patients enrolled.
Clinical and radiologic evaluations
Clinical data including name, sex, age, operative segments, operative duration, blood loss, postoperative ambulation time, days from surgery to discharge, postoperative complications, (preoperative, postoperative, and follow-up) Visual Analogue Scale (VAS) scores, (preoperative, postoperative, and followup) Oswestry Disability Index (ODI) scores, and MacNab criteria scores were recorded. The VAS was used to evaluate the back pain. The ODI was used to evaluate the daily life functions. The MacNab criteria were used to evaluate the patient satisfaction of surgery. The accuracy of screw implantation and complications such as screw loosening or breakage were also evaluated on the basis of radiologic data. Follow-up lumbar static and dynamic radiographs were used to affirm the fusion rate, and CT scans were obtained if necessary. The institutional ethics committee of Beijing Jishuitan Hospital approved the study. Written informed consent was obtained from all participants.
Surgical procedure
The same senior surgeon finished the operations. We used a three-dimensional (3D) fluoroscopy-based navigation system consisting of a modified C-arm CT system (ArcadisOrbic 3D; Siemens, Medical Solutions, Erlangen, Germany) and the workstation of navigation (The Stryker Spine Navigation System, version 1.2; Stryker, Missouri, MO, USA).
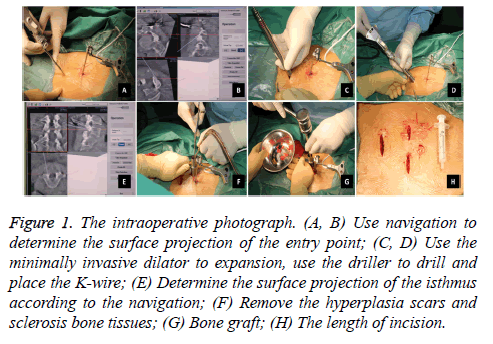
After general anaesthesia induction, the patients lied in a prone position. A C-arm was used for preoperative localization. Then, a tracker was placed on the spinous processes at the upper side of the target vertebra. Subsequently, ISO-C 3D was used to scan and automatically transfer the 3D images to the computer navigation workstation. After determining the surface projection of the entry point, the operator made a 3 cm transverse incision, cut the skin and subcutaneous tissue step by step, and used the minimally invasive dilator for expansion and the driller to drill. Under the guidance of 3D fluoroscopybased navigation, the operator completed the drilling and placed the K-wire. Subsequently, a 1.8 cm incision was made above the surface projection of the bilateral isthmus according to the navigation guidance. After gradually expanding, a diamond burr that has been calibrated to the navigation system was used to remove the hyperplasia scars and sclerosis bone tissues. Then, the bone grafts from the iliac were placed. The next step was to insert the hollow lag screws and apply pressure along with the K-wire. The last step was to wash and suture the wounds (Figure 1). No drainage was needed in this surgery. On the second day after the operation, the patients can walk and use a waist brace for 1 month.
Figure 1 : The intraoperative photograph. (A, B) Use navigation to determine the surface projection of the entry point; (C, D) Use the minimally invasive dilator to expansion, use the driller to drill and place the K-wire; (E) Determine the surface projection of the isthmus according to the navigation; (F) Remove the hyperplasia scars and sclerosis bone tissues; (G) Bone graft; (H) The length of incision.
Statistical analysis
SPSS17.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis; measurement data were described as x̄ ± s, whereas count data were described as percentage. Paired samples t-test was used for the comparison between groups for measurement data such as ODI and VAS, when they had a normal distribution. A nonparametric test was adopted when the data were not in accordance with the normal distribution. P<0.05 was considered significant.
Results
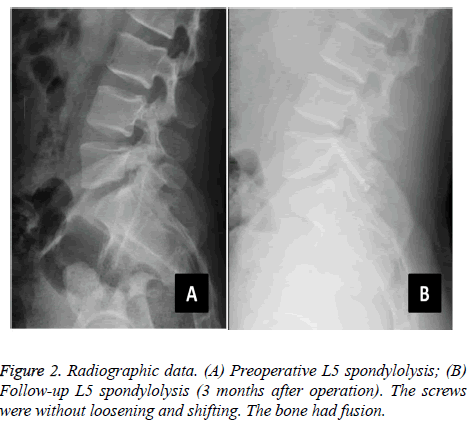
The average operative time was 97.8 ± 14.9 min, the average blood loss was 38.0 ± 15.6 ml, and the average postoperative hospital stay was 4.2 ± 0.9 days. The follow-up rate was 95.7% (22 of 23). One patient was lost owing to a change of telephone number. The follow-up duration was 24.2 months on average (range, 6-36 months). All wounds were well healed. The symptoms were significantly relieved after surgery, without wound infection, screw loosening, nerve damage, and other complications. The average time of returning to work/school was 3 weeks without strenuous exercises and hard physical labour. The postoperative and follow-up ODI (14.5 ± 9.3 and 10.6 ± 6.9, respectively) were significantly improved compared with the preoperative value (55.5 ± 16.3) (P<0.05). The followup ODI also showed significant improvement compared with the preoperative value (P=0.049) (Table 2). The postoperative and follow-up VAS (1.0 ± 1.5 and 0.7 ± 1.2, respectively) was significantly improved compared with that before surgery (5.5 ± 1.3) (P<0.05), whereas the comparisons of VAS between postoperative and follow-up values showed no significant difference (P=0.121) (Table 2). In the MacNab criteria, 20 cases were excellent, whereas 2 cases were good. We used the method of Andrew to evaluate the accuracy of isthmus screw implantation [24]. On the basis of the postoperative CT scan, we divided the degree of the screw implantation into excellent, good, moderate, and poor. All the 22 patients were implanted with a total of 44 isthmus screws. The rate of screw implantation was excellent (100%, 44 of 44), with no misplacement or clinical deficits. The isthmus solid fusion rate was 81.8% (36 of 44) according to the follow-up radiographs and CT scans (Figure 2). For the four patients with non-union, the slight back pain had no influence on their daily lives.
| Preoperative | Postoperative | Follow-up | |
|---|---|---|---|
| ODI | 55.5±16.3** | 14.5±9.3* | 10.6±6.9* |
| VAS | 5.5±1.3** | 1.1±1.5* | 0.7±1.2* |
**Comparison in ODI and VAS scores. There is a significant difference between preoperative and postoperative ODI and VAS scores (P<0.05); there is a significant difference between preoperative and follow-up ODI and VAS scores (P<0.05).
*Comparison in ODI and VAS scores. There is a significant differences between postoperative and follow-up ODI (P<0.05)and no significant on VAS scores (P>0.05).
Table 2. The ODI and VAS scores of preoperative, postoperative and follow-up.
Discussion
Patients with spondylolysis who had failed conservative treatment should consider surgical treatment. The surgical approach has developed from multi-segment fusion to internal segment fusion. As many researchers have noticed that multisegment fusion reduces the range of motion and accelerates the degeneration of adjacent segments [25-28], the internalsegment fusion technique has been considered a better choice. Kimura first used the bone graft method in isthmus defects to treat lumbar spondylolysis [29]. Buck reported a study on the combination of isthmus screw fixation and the bone graft method [11]. Since then, a variety of effective methods have been reported, such as the tension band method reported by Noil et al. [30], as well as the nail hook method reported by Morscher et al. [31]. However, the Scott et al. methods are insufficient in many aspects, such as causing more serious stripping on soft tissues, more bleeding, and a higher risk of nerve injury [32]. The Scott method demands cutting off the iliolumbar ligament to expose the transverse process during the operation, which may cause vertebral instability [33]. In addition, these two methods may result in bone absorption; implant loosening, or even back pain owing to the greater tension [34].
Compared with other methods, the Buck method is the most direct way to treat fatigue fracture of the isthmus by using lag screws fixed across the fracture line. Biomechanical tests have shown that this method is a better choice for fixation. Fan et al. indicated that the Buck method can effectively stabilize the isthmus [35]. Deguchi et al. found that the Buck method is the best technique in terms of a fixed intensity in biomechanical tests compared with the other methods [36]. However, because of the greater difficulty in operation and the possible nerve root damage caused by misplacement of the isthmus screws, the Buck method has the limitation of a longer learning curve [37].
Since its application in the field of spine surgery in the 1990’s, the navigation technology has been well developed [38]. The real-time navigation system can help the surgeon pinpoint a fixed orbit and greatly improve the accuracy of screw implantation, especially for those with complex anatomical structures and a high-risk surgical site [39]. The navigation technology can accomplish percutaneous screw implantation and achieve minimally invasive surgery. As it has the combination of navigation technology and minimal invasiveness, the CAMISS technique has been applied in many areas of spinal surgery.
Compared with the traditional Buck method, CAMISS has many advantages. First, the navigation system can achieve the precise location during the surgery and ensure that the lag screws cross the isthmus stump successfully. It can reduce both the risk of surgery and the possibility of nerve injury. In this study, no serious screw misplacement was found and the solid fusion rate was close to those in previous reports. Moreover, there were no postoperative complications, and the ODI and VAS scores improved significantly. Second, minimally invasive surgery can maximize the retention of the back muscles and soft tissues, accelerating postoperative recovery and reducing the chronic back pain caused by extensive dissection. In our study, the amount of blood loss was 37.5 ml, which is largely lower than that of the traditional Buck method or other surgical approaches. This result is similar to another minimally invasive method by Brennan et al. [40]. Furthermore, patients do not need to undergo postoperative drainage and are able to ambulate the next day after the surgery. Patients have reported higher satisfaction with this surgery. Third, the CAMISS method can avoid repeated intraoperative fluoroscopy and reduce the radiation damage to the operators. Finally, this method is easy to comprehend fully, which can reduce the learning curve for junior doctors. Lee et al. compared between direct repair surgical treatment and conservative treatment for young patients with spondylolysis [41]. They found that direct repair surgery had the same treatment effect but more complications. To overcome the shortcomings of the direct repair method, we used the CAMISS method, which not only improves the treatment effects but also reduces complications.
In summary, the CAMISS-Buck technique has the advantages of both navigation technology and minimal invasiveness, and can overcome the shortcomings of the traditional Buck method. This method shows several benefits, including a higher accuracy, less intraoperative blood loss, and earlier rehabilitation. However, our study has some limitations in the sample number and follow-up duration. In the future, a study with a larger size and longer follow-up may be considered to compare the effect among the CAMISS-Buck method, traditional Buck method, and conservative treatment of spondylolysis. Nevertheless, the CAMISS-Buck method is an effective and promising surgical method.
Conflicts of Interest
All of the authors declare that they have no conflicts of interest regarding this paper.
References
- Lim MR, Yoon SC, Green DW. Symptomatic spondylolysis: diagnosis and treatment. CurrOpinPediatr 2004; 16: 37-46.
- Lundin DA, Wiseman D, Ellenbogen RG, Shaffrey CI. Direct repair of the pars interarticularis for spondylolysis and spondylolisthesis. PediatrNeurosurg 2003; 39: 195-200.
- Farfan HF, Osteria V, Lamy C. The mechanical etiology of spondylolysis and spondylolisthesis. ClinOrthopRelat Res 1976; 117: 40-55.
- Wiltse LL, Widell EH Jr, Jackson DW. Fatigue fracture: the basic lesion is inthmicspondylolisthesis. J Bone Joint Surg Am 1975; 57: 17-22.
- Cyron BM, Hutton WC, Troup JD. Spondylolytic fractures. J Bone Joint Surg Br 1976; 58-58B: 462-6.
- Cyron BM, Hutton WC. The fatigue strength of the lumbar neural arch in spondylolysis. J Bone Joint Surg Br 1978; 60-60B: 234-8.
- Kurd MF, Patel D, Norton R, Picetti G, Friel B. Non-operative treatment of symptomatic spondylolysis. J Spinal Disord Tech 2007; 20: 560-564.
- Willner S. Effect of a rigid brace on back pain. ActaOrthopScand 1985; 56: 40-42.
- Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for paediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long J Neurosurg Spine 2012; 16: 610-614.
- Winter RB. The natural history of spondylolysis and spondylolisthesis. J Bone Joint Surg Am 1985; 67: 823.
- Buck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br 1970; 52: 432-437.
- Giudici F, Minoia L, Archetti M, Corriero AS, Zagra A. Long-term results of the direct repair of spondylolisthesis. Eur Spine J 2011; 20: 115-120.
- Reitman CA, Esses SI. Direct repair of spondylolytic defects in young competitive athletes. Spine J 2002; 2: 142-144.
- Drazin D, Shirzadi A, Jeswani S, Ching H, Rosner J, Rasouli A, Kim T, Pashman R, Johnson JP. Direct surgical repair of spondylolysis in athletes: indications, techniques, and outcomes. Neurosurg Focus 2011; 31: E9.
- Kim YT, Lee H, Lee CS, Lee DH, Hwang CJ. Direct repair of the pars interarticularis defect in spondylolysis. J Spinal Disord Tech 2012.
- Prasartritha T. Surgical repair of pars defects in spondylolysis. J Med Assoc Thai 2001; 84: 1235-1240.
- Raley DA, Mobbs RJ. Retrospective computed tomography scan analysis of percutaneously inserted pedicle screws for posterior transpedicular stabilization of the thoracic and lumbar spine: accuracy and complication rates. Spine 2012; 37: 1092-1100.
- Weng C, Tian W, Li ZY, Liu B, Li Q, Wang YQ, Sun YZ. Surgical management of symptomatic osodontoideum with posterior screw fixation performed using the magerl and harms techniques with intraoperative 3-dimensional fluoroscopy-based navigation. Spine 2012; 37: 1839-1846.
- Tian W, Weng C, Liu B, Li Q, Hu L, Li ZY, Liu YJ, Sun YZ. Posterior fixation and fusion of unstable Hangmans fracture by using intraoperative three-dimensional fluoroscopy-based navigation. Eur Spine J 2012; 21: 863-871.
- Tian W, Lang Z. Placement of pedicle screws using three-dimensional fluoroscopy-based navigation in lumbar vertebrae with axial rotation. Eur Spine J 2010; 19: 1928-1935.
- Tian W, Xu YF, Liu B, Liu YJ, He D, Yuan Q, Lang Z, Han XG. Computer-assisted minimally invasive transforaminal lumbar interbody fusion may be better than open surgery for treating degenerative lumbar disease. J Spinal Disord Tech 2014.
Wald JT, Geske JR, Diehn FE, Murthy NS, Kaufmann TJ, Thielen KR, Morris JM, Lehman VT, Maus TP. A practice audit of CT-guided injections of pars interarticularis defects in patients with axial low back pain: a primer for further investigation. Pain Med 2014; 15: 745-750. - Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001; 26: 1873-1878.
- Youkilis AS, Quint DJ, McGillicuddy JE, Papadopoulos SM. Stereotactic navigation for placement of pedicle screws in the thoracic spine. Neurosurgery 2001; 48: 771-778.
- Axelsson P, Johnsson R, Strömqvist B. The spondylolytic vertebra and its adjacent segment. Mobility measured before and after posterolateral fusion. Spine1997; 22: 414-417.
- Mihara H, Onari K, Cheng BC, David SM, Zdeblick TA. The biomechanical effects of spondylolysis and its treatment. Spine 2003; 28: 235-238.
- Cheh G, Bridwell KH, Lenke LG, Buchowski JM, Daubs MD, Kim Y, Baldus C. Adjacent segment disease followinglumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine 2007; 32: 2253-2257.
- Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine 2004; 29: 1938-1944.
- Kimura M. My method of filing the lesion with spongy bone in spondylolysis and spondylolistesis. SeikeiGeka 1968; 19: 285-296.
- Nicol RO, Scott JH. Lytic spondylolysis. Repair by wiring. Spine 1986; 11: 1027-1030.
- Morscher E, Gerber B, Fasel J. Surgical treatment of spondylolisthesis by bone grafting and direct stabilization of spondylolysis by means of a hook screw. Arch Orthop Trauma Surg 1984; 103: 175-178.
- Debusscher F, Troussel S. Direct repair of defects in lumbar spondylolysis with a new pedicle screw hook fixation: clinical, functional and Ct-assessed study. Eur Spine J 2007; 16: 1650-1658.
- Aihara T, Takahashi K, Yamagata M, Moriya H, Tamaki T. Biomechanical functions of the iliolumbar ligament in L5 spondylolysis. J OrthopSci 2000; 5: 238-242.
- Ulibarri JA, Anderson PA, Escarcega T, Mann D, Noonan KJ. Biomechanical and clinical evaluation of a novel technique for surgical repair of spondylolysis in adolescents. Spine 2006; 31: 2067-2072.
- Fan J, Yu GR, Liu F, Zhao J, Zhao WD. A biomechanical study on the direct repair of spondylolysis by different techniques of fixation. OrthopSurg 2010; 2: 46-51.
- Deguchi M, Rapoff AJ, Zdeblick TA. Biomechanical comparison of spondylolysis fixation techniques. Spine1999; 24: 328-333.
- Tonino A, van der Werf G. Direct repair of lumbar spondylolysis. 10-year follow-up of 12 previously reported cases. ActaOrthopScand 1994; 65: 91-93.
- Amiot LP, Labelle H, DeGuise JA, Sati M, Brodeur P. Computer-assisted pedicle screw fixation. A feasibility study. Spine 1995; 20: 1208-1212.
- Holly LT, Foley KT. Intraoperative spinal navigation. Spine 2003; 28: 54-61.
- Brennan RP, Smucker PY, Horn EM. Minimally invasive image-guided direct repair of bilateral L-5 pars interarticularis defects. Neurosurg Focus 2008; 25: E13.
- Lee GW, Lee SM, Ahn MW, Kim HJ, Yeom JS. Comparison of surgical treatment with direct repair versus conservative treatment in young patients with spondylolysis: a prospective, comparative, clinical trial. Spine J 2015; 15: 1545-1553.