ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 20
Detection of serum immune factors in children with hand-foot-and-mouth disease (HFMD) and the clinical significance
Department of Pediatrics, Zhoushan Maternal and Child Health Care Hospital, Zhoushan, Zhejiang, PR China
- *Corresponding Author:
- Qiao Xu
Department of Pediatrics
Zhoushan Maternal and Child Health Care Hospital, PR China
Accepted date: September 25, 2017
Objective: To detect serum immune factors in children with hand-foot-and-mouth disease (HFMD), and to explore the clinical significance.
Methods: A total of 28 severe hand-foot-and-mouth disease (HFMD) patients with enterovirus 71 (EV71) infection, 37 mild HFMD patients with EV71 infection, and 23 mild HFMD patients with coxsackievirus A16 (CVA16) infection were selected from January 2015 to January 2017 in XX hospital. At the same time, 25 healthy people were also selected as control. Non-anticoagulated venous blood (2 mL) was collected from each patient before treatment, 3 days after the beginning of treatment, and on the day of discharge. Serum levels of IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 were detected by Enzyme-Linked Immunosorbent Assay (ELISA).
Results: Significant higher levels of serum IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 were found in all HFMD patients compared with control (p<0.01). Highest serum levels of IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 were found in severe HFMD patients with EV71 infection. No significant differences in levels of IL-8, IL-12 and IL-18 were found between HFMD patients with EV71 infection and mild HFMD patients with CVA16 infection. With treatment, levels of IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 decreased gradually to reach normal level. Serum levels of TNF-α, INF-γ, IL-8, IL-12 and IL-18 were all positively correlated with serum level of IgM.
Conclusion: Serum immune factors were involved in the development of HFMD.
Keywords
Hand-foot-and-mouth disease, Serum immune factors, Enterovirus 71, Coxsackievirus A16.
Introduction
As a common enteroviral disease, hand-foot-and-mouth disease (HFMD) usually affects children under 5 years old and infants over 6 months [1]. The majority of cases of HFMD were from in Asian countries, especially China. Between 2008 and 2014, about 12 million new cases of HFMD were reported in China, bringing a heavy burden for health care systems across the country [2]. This disease can be spread through droplets or excreta. Although HFMD is benign in most cases, an outbreak of a subtype of HFMD in Shandong province of China causes a large number of deaths in a short time [3]. Therefore, the prevention, early diagnosis and treatment of HFMD have become a hot research topic in the field of public health.
As an infectious disease, HFMD is caused by the infection of human enterovirus 71 (EV71) and coxsackievirus A16 (CVA16), and in some rare cases, by other enteroviruses [4]. Compared with CVA16, EV71 usually can lead to more severe conditions [5], indicating the different pathogenesis of HFMD caused by different viral infections. However, the molecular mechanism is still unclear. Serum immune factors are closely correlated with the development of HFMD. It has been reported that IL-8 and IL-10 are differentially expressed in patients with severe and mild HFMD [6]. In another, different expression pattern of immune factors were also found in HFMD patients with CVA16 or EV71 infection [7]. However, a systemic analysis on the expression patterns of immune factors in different types of HFMD caused by different viral infection still hasn't been reported.
In this study, patients with severe or mild HFMD caused by EV71 or CVA16 infection were included. Levels of serum immune factors including IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 were detected in different groups of patients at different time points. In addition, the correlation between serum levels of IgM and serum levels of TNF-α, INF-γ, IL-8, IL-12 and IL-18 were also explored.
Materials and Methods
Patients
Twenty-eight severe hand-foot-and-mouth disease (HFMD) patients with enterovirus 71 (EV71) infection, 37 mild HFMD patients with EV71 infection, and 23 mild HFMD patients with coxsackievirus A16 (CVA16) infection were selected from January 2015 to January 2017 in XX hospital. Inclusion criteria: (1) between 0 and 14 years old; (2) diagnosed with HFMD; (3) with CVA16 or EV71 infection confirmed by laboratory examination; (4) patients who were willing to provide serum samples. Patient with other enteroviruses or bacterial infections were not included. Patients with primary heart disease and severe underlying disease were also not included. Diagnostic criteria: (1) mild HFMD, patients with oral ulcers, and hands, feet, knees and buttocks blisters, no central nervous system or respiratory symptoms were observed; (2) severe HFMD, stage II-encephalitis, muscle clonic seizure and vomiting, stage III-encephalitis complications, tachycardia, hyperglycemia, polypnea or hypertension, stage IV-cardiopulmonary failure complications, tissue hypoxia, hypotension, shock, pulmonary edema, or bleeding. All patients with CVA16 infection showed mild HFMD. This study was approved by the Ethics Committee of our hospital, all patients and their family members signed informed consent.
Treatments
All patients were asked to take plenty of cool fluids (ice cream or flavored ice pops) to improve the conditions of sore throat. Spicy and acidic foods and drinks were avoided. For patients with fever and pain, regular strength Tylenol® tablets or Advil tablet was provided with a dose of 1 tablet per time and 3 times per day. Most patients recovered within 1 week, all patients recovered within two weeks.
Specimen collection
Non-anticoagulated venous blood (2 mL) was collected from each patient before treatment, 3 days after the beginning of treatment, and on the day of discharge. The same amount of venous blood was also collected from each control. After 2 h at room temperature, blood samples were centrifuged at 1000 rpm for 20 min to collect serum. Serum was transferred to EP tube stored at -80°C.
Enzyme-Linked Immunosorbent Assay (ELISA)
Serum level of IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 were detected by ELISA using a microplate reader (BIO-RAD 680, CA, USA). ELISA kits of IgM (ab137982), IL-8 (ab46032), IL-12 (ab46035) and IL-18 (ab215539) were from Abcam (Combridge, UK), and kits of TNF-α and INF-γ were from R&D systems (USA).
Statistical analysis
SPSS19.0 software was used for all statistical analyses. Measurement data were expressed as mean ± standard deviation, and comparisons between groups were performed using t test. p<0.05 was considered to be statistically significant.
Results
Comparison of general information among groups
As shown in Table 1, no significant differences in age, gender and weight were found among three groups of patients (p>0.05).
| a n=28 | b n=37 | c n=23 | d n=25 | |
|---|---|---|---|---|
| Age | 3.17 (1.24) | 3.46 (0.98) | 3.23 (2.13) | 3.38 (0.76) |
| Gender | ||||
| Male | 15 | 17 | 11 | 13 |
| Female | 13 | 20 | 12 | 12 |
| Weight | 16.31 (5.85) | 16.13 (6.46) | 16.43 (4.87) | 15.98 (5.14) |
Table 1: Comparison of general information among groups.
Comparison of serum immune factors among groups
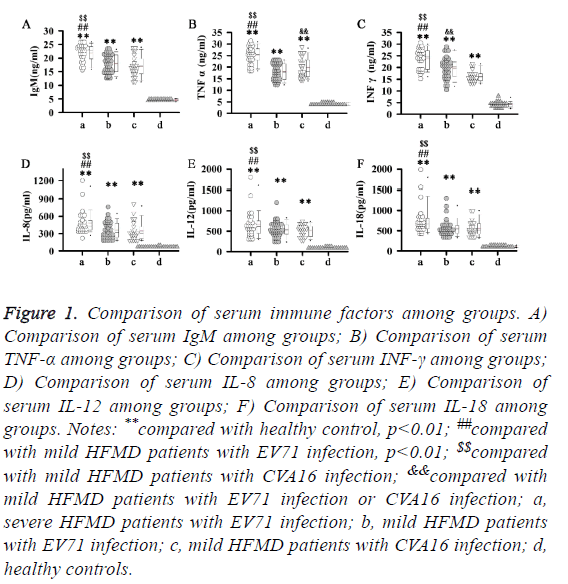
At the earliest response factor of immune response, IgM level is closely correlated with the severity of viral infection. In our study, significant higher levels of IgM were found in all HFMD patients compared with control group (p<0.01). In addition, highest levels of IgM were found in severe HFMD patients with EV71 infection. No significant difference in level of IgM was found between mild HFMD patients with EV71 infection and mild HFMD patients with CVA16 infection. Similar results were found in serum levels of TNF-α, INF-γ, IL-8, IL-12 and IL-18. Significant higher levels of TNF-α, INF-γ, IL-8, IL-12 and IL-18 were found in all HFMD patients compared with control group (p<0.01). In addition, highest levels of TNF-α, INF-γ, IL-8, IL-12 and IL-18 were found in severe HFMD patients with EV71 infection. No significant difference in level of IL-8, IL-12 and IL-18 was found between mild HFMD patients with EV71 infection and mild HFMD patients with CVA16 infection. Significant higher level of TNF-α was found in mild HFMD patients with CVA16 infection than in mild HFMD patients with EV71 infection, but serum level of INF-γ was higher in mild HFMD patients with EV71 infection compared with that in mild HFMD patients with CVA16 infection. Those data suggest that different immune response pathways are involved in different types of HFMD (Figure 1).
Figure 1: Comparison of serum immune factors among groups. A) Comparison of serum IgM among groups; B) Comparison of serum TNF-α among groups; C) Comparison of serum INF-γ among groups; D) Comparison of serum IL-8 among groups; E) Comparison of serum IL-12 among groups; F) Comparison of serum IL-18 among groups. Notes: **compared with healthy control, p<0.01; ##compared with mild HFMD patients with EV71 infection, p<0.01; $$compared with mild HFMD patients with CVA16 infection; &&compared with mild HFMD patients with EV71 infection or CVA16 infection; a, severe HFMD patients with EV71 infection; b, mild HFMD patients with EV71 infection; c, mild HFMD patients with CVA16 infection; d, healthy controls.
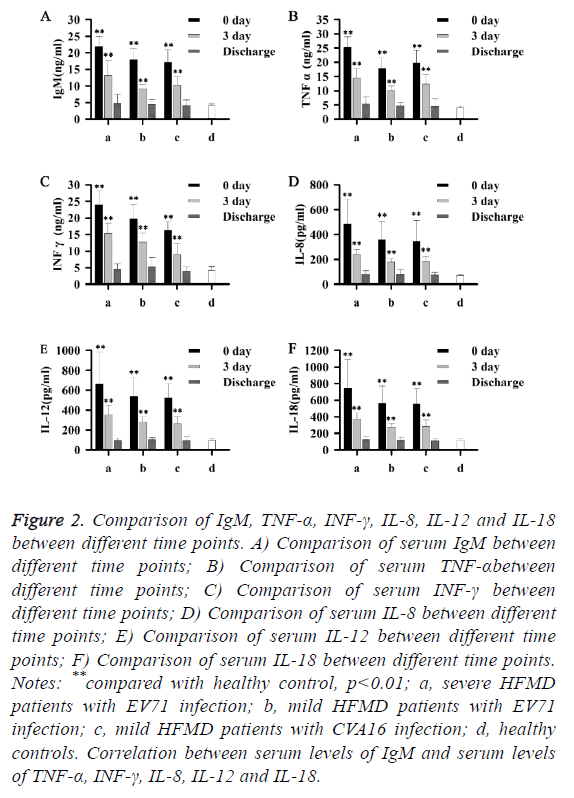
As shown in Figure 2, with treatment, serum levels of IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 gradually decreased, and almost reached normal levels on the day of discharge. Those data suggest that levels of IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 were negatively correlated with the treatment outcomes of patients with HFMD.
Figure 2: Comparison of IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 between different time points. A) Comparison of serum IgM between different time points; B) Comparison of serum TNF-αbetween different time points; C) Comparison of serum INF-γ between different time points; D) Comparison of serum IL-8 between different time points; E) Comparison of serum IL-12 between different time points; F) Comparison of serum IL-18 between different time points. Notes: **compared with healthy control, p<0.01; a, severe HFMD patients with EV71 infection; b, mild HFMD patients with EV71 infection; c, mild HFMD patients with CVA16 infection; d, healthy controls. Correlation between serum levels of IgM and serum levels of TNF-α, INF-γ, IL-8, IL-12 and IL-18.
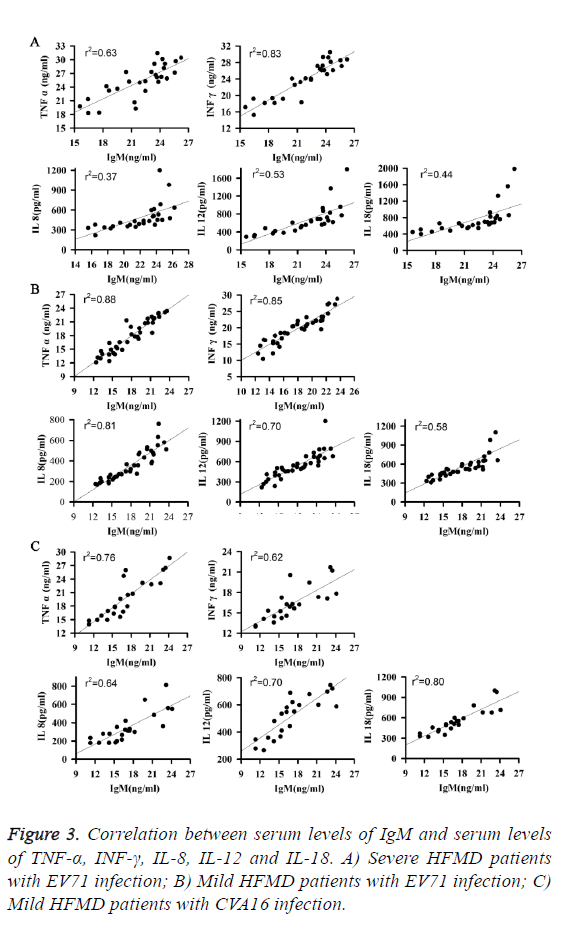
As shown in Figure 3, serum levels of TNF-α, INF-γ, IL-8, IL-12 and IL-18 were all positively correlated with serum level of IgM at all three groups of patients. Those results suggest that the increased level of serum IgM may be a subsequence of increase serum levels of TNF-α, INF-γ, IL-8, IL-12 and IL-18 in patients with HFMD.
Discussion
Clinical studies have shown that enteroviruses infection, especially subtype EV71 and CVA16, is the main cause of HFMD [8]. HEMD is usually self-limited. However, in some extreme cases, EV71 may lead to severe symptoms or even death [9]. During an outbreak of HFMD in 1998 in Taiwan, EV71 infection was reported to be responsible for 78 cases of deaths among 129,000 patients diagnosed with HFMD [10]. In the main land of China, EV71 infection was proved to be correlated with 2457 deaths of more than 7 million new cases of HFMD [11]. In our study, a total of 65 HFMD patients with EV71 infection were included. Among them, severe HMD was observed in 28 cases, accounting for 43% of all the cases. In contrast, all 25 HFMD patients with CVA16 infection showed mild symptoms. Our study further confirmed that EV71 infection in the main cause of severe HFMD.
Immunoglobulin M, or IgM, is the largest antibody produced by vertebrates. IgM is also the first antibody to appear in the initial response of the exposure to antigens, such as viral infection [12]. Therefore, changes in levels of IgM can be used to sensitively reflect the conditions of viral infection. A recent study carried out by Chan et al. has shown that IgM is involved in the development of HFMD caused by EV71 [13]. Besides that, changes in levels of IgM were also detected in adults with CVA16 infection [14]. In another study, HEV71 and CVA16 specific IgM antibodies have been proved to be effective and accurate in the diagnosis of HFMD [15]. In this study, significantly increased levels of IgM were detected in all patients with HFMD compared with healthy controls, further confirming that IgM is involved in the development of HFMD caused by both HEV71 and CVA16. In addition, levels of IgM was found to be significant higher in severe HFMD patients with EV71 infection compared with mild HFMD patients with EV71 infection or mild HFMD patients with CVA16 infection. However, no significant differences in levels of IgM were found between mild HFMD patients with EV71 infection and mild HFMD patients with CVA16 infection. Those results suggest that level of IgM in HFMD patients is positively correlated with the severity of disease, but may be not correlated with the types of viral infection.
It has been proved that the profile of cytokines has certain effects on the severity and pathogenicity of HFMD caused by EV71 [16]. In another study, changes in the expression of cytokines cascade in patients HFMD were proved to be able to induce immune response to CV-A16 and EV-71 infection [17]. In this study, levels of TNF-α, INF-γ, IL-8, IL-12 and IL-18 were significant higher in patients with HFMD compared with healthy controls. With treatment, serum level of TNF-α, INF-γ, IL-8, IL-12 and IL-18 gradually decreased to normal levels. Those results suggest that TNF-α, INF-γ, IL-8, IL-12 and IL-18 were involved in the pathogenesis of HFMD, and the levels of those cytokines can be used to predict the treatment outcomes. Besides that, no significant difference in level of IL-8, IL-12 and IL-18 was found between mild HFMD patients with EV71 infection and mild HFMD patients with CVA16 infection, indicating that levels of IL-8, IL-12 and IL-18 were only correlated with the severity of disease, but may be not correlated with the types of viral infection. We also found that serum TNF-α level was significant higher, but serum level of INF-γ was significant lower in HFMD patients with CVA16 infection than in mild HFMD patients with EV71 infection. It has been proved that, CVA16 infection can induce the production of TNF-α in congenital immune cells through TLR3-TRIF signaling pathway [18]. Both previous studies and the findings in this study suggest different immune response pathways involved in different types of HFMD. The production of IgM is affected by the levels of various cytokines in the body [19]. For example, it has been reported that IFN-γ produced by NK and T cells can promote the secretion of IL-6 by B cells, which in turn induce the secretion of IgM [20]. In another study, IL-12 was found to be able to inhibit the production of lgE but promote the production of IgM by human peripheral blood mononuclear cells [21]. In this study, serum levels of TNF-α, INF-γ, IL-8, IL-12 and IL-18 were all positively correlated with serum level of IgM at all three groups of patients, indicating that the increased level of serum IgM may be a subsequence of increase serum levels of those cytokines in patients with HFMD.
In conclusion, levels of serum IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 were increased in HFMD patients compared with control group. Highest serum levels of IgM, TNF-α, INF-γ, IL-8, IL-12 and IL-18 were found in severe HFMD patients with EV71 infection. No significant differences in levels of IL-8, IL-12 and IL-18 were found between HFMD patients with EV71 infection and mild HFMD patients with CVA16 infection, but TNF-α and INF-γ showed different expression pattern in patient with EV71 or CVA16 infection. So we think serum immune factors were involved in the development of different types of HFMD through different pathways.
Acknowledgment
We thankful for the financial support from Zhoushan Zhejiang science and Technology Bureau: General non marine projects; (2011C13046).
References
- Reich D, Psomadakis CE, Buka B. Top 50 Dermatology Case Studies for Primary Care. Springer, Berlin, 2017.
- Van Boeckel TP, Takahashi S, Liao Q. Hand, foot, and mouth disease in China: critical community size and spatial vaccination strategies. Scientific Rep 2016.
- Zhang Y, Tan XJ, Wang HY. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol 2009; 44: 262-267.
- Guan H, Wang J, Wang C. Etiology of multiple Non-EV71 and non-CVA16 enteroviruses associated with hand, foot and mouth disease in Jinan, China, 2009-June 2013. PloS One 2015; 10: e0142733.
- Huang CC, Liu CC, Chang YC. Neurologic complications in children with enterovirus 71 infection. N Eng J Med 1999; 341: 936-942.
- Han J, Wang Y, Gan X. Serum cytokine profiles of children with human enterovirus 71-associated hand, foot, and mouth disease. J Med Virol 2014; 86: 1377-1385.
- Zhang SY, Xu MY, Xu HM. Immunologic characterization of cytokine responses to enterovirus 71 and coxsackievirus a16 infection in children. Medicine 2015.
- Huang WC, Huang LM, Lu CY. Atypical hand-foot-mouth disease in children: a hospital-based prospective cohort study. Virol J 2013; 10: 209.
- Lin TY, Chang LY, Hsia SH. The 1998 enterovirus 71 outbreak in Taiwan: pathogenesis and management. Clin Infect Dis 2002; 34: S52-S57.
- Ho M, Chen ER, Hsu KH. An epidemic of enterovirus 71 infection in Taiwan. N Eng J Med 1999; 341: 929-935.
- Liang Z, Wang J. EV71 vaccine, an invaluable gift for children. Clin Translat Immunol 2014; 3: e28.
- Villar LM, Casanova B, Ouamara N. Immunoglobulin M oligoclonal bands: biomarker of targetable inflammation in primary progressive multiple sclerosis. Annal Neurol 2014; 76: 231-240.
- Chan YF. Immunodominant IgM and IgG epitopes recognized by antibodies induced in enterovirus A71-associated hand, foot and mouth disease patients. 2016.
- Legay F, Lévêque N, Gacouin A. Fatal coxsackievirus A-16 pneumonitis in adult. Emerg Infect Dis 2007; 13: 1084.
- Yu N, Guo M, He SJ. Evaluation of human enterovirus 71 and coxsackievirus A16 specific immunoglobulin M antibodies for diagnosis of hand-foot-and-mouth disease. Virology J 2012; 9: 12.
- Han J, Wang Y, Gan X. Serum cytokine profiles of children with human enterovirus 71-associated hand, foot, and mouth disease. J Med Virol 2014; 86: 1377-1385.
- Zhang SY, Xu MY, Xu HM. Immunologic characterization of cytokine responses to enterovirus 71 and coxsackievirus a16 infection in children. Med 2015.
- Yang J, Yang C, Guo N. Type I Interferons Triggered through the Toll-Like Receptor 3–TRIF Pathway Control Coxsackievirus A16 Infection in Young Mice. J Virol 2015; 89: 10860-10867.
- Tangye SG, Ferguson A, Avery DT. Isotype switching by human B cells is division-associated and regulated by cytokines. J Immunol 2002; 169: 4298-4306.
- Yi AK, Chace JH, Cowdery JS. IFN-gamma promotes IL-6 and IgM secretion in response to CpG motifs in bacterial DNA and oligodeoxynucleotides. J Immunol 1996; 156: 558-564.
- De Boer BA, Kruize YC, Rotmans PJ. Interleukin-12 suppresses immunoglobulin E production but enhances immunoglobulin G4 production by human peripheral blood mononuclear cells. Infect Immunity 1997; 65: 1122-1125.