ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2016) Volume 27, Issue 4
Demonstration of a chronic myelogenous leukemia clone in acute myelogenous leukemia
Byung Ryul Jeon1, Se Hyung Kim2, Sung Kyu Park2, Rojin Park3, Dae Sik Hong2 and You Kyoung Lee1*
1Department of Laboratory Medicine and Genetics, Soonchunhyang University College of Medicine, Bucheon, Korea
2Division of Hematology and Oncology, Department of Internal Medicine, Soonchunhyang University College of Medicine, Bucheon, Korea
3Department of Laboratory Medicine, Soonchunhyang University College of Medicine, Seoul, Korea
- *Corresponding Author:
- You Kyoug Lee
Department of Laboratory Medicine and Genetics, Soonchunhyang University, Korea
E-mail: cecilia@schmc.ac.kr
Accepted on March 30, 2016
In the present study, we report a case of a 58-year-old female patient presenting with Philadelphiachromosome (Ph)-negative blast cells and Ph -positive mature cells at diagnosis. The complete blood cell count showed the patient had a white blood cell count of 35.5 × 109/L and a differential blast count of 62%. A bone marrow biopsy showed closely packed marrow with blast cell infiltration. Blasts were weakly positive for MPO stain, but negative for PAS and ANAE stains. Moreover, blasts tested positive for CD34, CD13, CD117, CD15, CD33, and CD7 and negative for TdT, CD3, CD22, and CD19 in cytometric immunophenotyping experiments. The patient, who had the karyotype 46,XX,t(9;22) (q34;q11.2) [14]/45,XX,inv(3)(q21q26.2),-7[4], was therefore diagnosed as having AML. Fluorescence in situ hybridization (FISH) showed a decrease in Ph-positive clones and an increase in monosomy 7- positive clones after CD34-positive cell enrichment, suggesting that the blasts were primarily Phnegative. This case highlights the necessity of analyzing the cytogenetic characteristics of blasts to better understand the nature of the disease when only a fraction of the cells are Ph-positive.
Keywords
AML, CML, BCR/ABL1, CD 34 enrichment, Monosomy 7.
Introduction
The BCR/ABL1 rearrangement, the genetic hallmark of chronic myelogenous leukemia (CML), also appears as a distinct entity in mixed-phenotype acute leukemia and B lymphoblastic leukemia/lymphoma in the revised World Health Organization (WHO) classification. Although not defined in the revised WHO classification, Philadelphia chromosomepositive (Ph+) acute myeloid leukemia (AML) is also considered a rare entity with a poor prognosis [1,2].
In addition, AML with BCR/ABL1-positive subclones and CML with increased numbers of Philadelphia-chromosomenegative blasts during treatment have also been reported [3,4]. To our knowledge, however, no study has identified a Philadelphia-chromosome-positive CML clone with increased numbers of blasts without the Philadelphia chromosome suggesting AML at diagnosis. In this study, we report a case of AML, which has Philadelphia-chromosome-negative blasts and Philadelphia-chromosome-positive mature cells at diagnosis.
Case Report
A 58-year-old female was admitted to our hospital because of a pale appearance, which began about 20 days before admission.
She had been diagnosed with schizophrenia about 40 years previously and had taken drugs occasionally since then, but no treatment record was available. The patient and her family had no memory of hematological abnormalities. A physical examination showed pale conjunctivae and splenomegaly. The complete blood cell (CBC) count revealed a white blood cell (WBC) count of 35.5×109 /L with a differential count of 62% blasts, 27% neutrophils, 6% bands, 2% metamyelocytes, 2% lymphocytes, and 1% monocytes (Figure 1A). The hemoglobin was 47 g/L and the platelet count was 30×109/L.
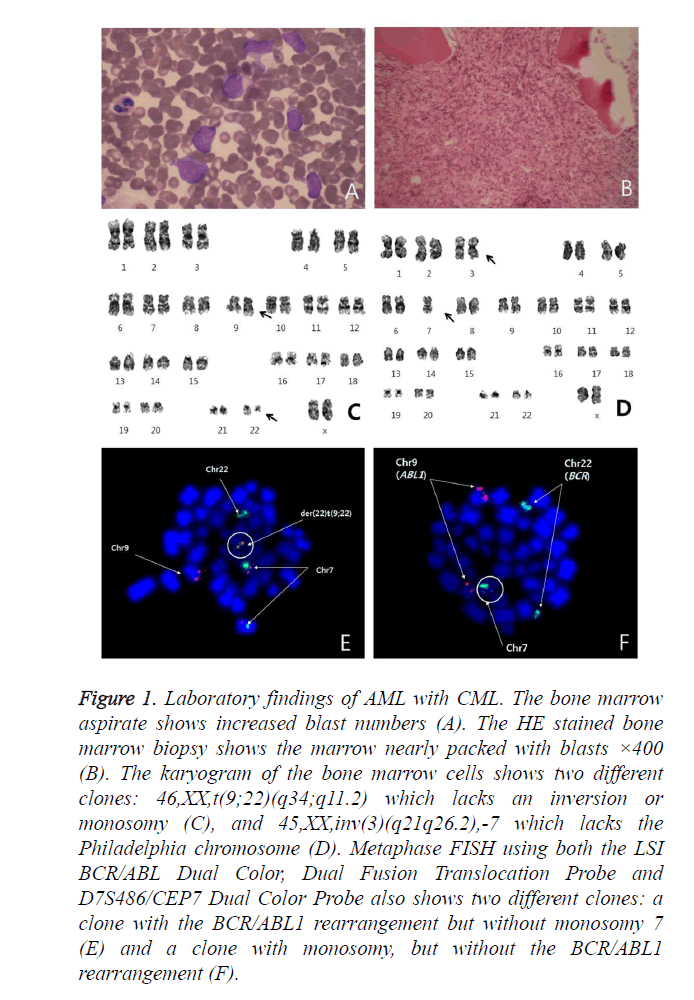
Figure 1. Laboratory findings of AML with CML. The bone marrow aspirate shows increased blast numbers (A). The HE stained bone marrow biopsy shows the marrow nearly packed with blasts ×400 (B). The karyogram of the bone marrow cells shows two different clones: 46,XX,t(9;22)(q34;q11.2) which lacks an inversion or monosomy (C), and 45,XX,inv(3)(q21q26.2),-7 which lacks the Philadelphia chromosome (D). Metaphase FISH using both the LSI BCR/ABL Dual Color, Dual Fusion Translocation Probe and D7S486/CEP7 Dual Color Probe also shows two different clones: a clone with the BCR/ABL1 rearrangement but without monosomy 7 (E) and a clone with monosomy, but without the BCR/ABL1 rearrangement (F).
A bone marrow (BM) biopsy showed nearly packed marrow with infiltration of blast cells (Figure 1B). Flow cytometric immunophenotyping studies showed that the blasts were positive for CD34, CD13, CD117, CD15, CD33, and CD7 and negative for TdT, CD 3, CD22, and CD19.
Conventional cytogenetic analysis was performed using heparinized BM samples. The patient’s karyotype was 46,XX,t(9;22)(q34;q11.2)[14]/45,XX,inv(3)(q21q26.2),-7[4] (Figures 1C and 1D). Fluorescence in situ hybridization (FISH) analysis was performed on both BM and peripheral blood (PB) samples using the LSI BCR/ABL Dual Color, Dual Fusion Translocation Probe and D7S486/CEP7 Dual Color Probe (Vysis/Abbott Molecular, Des Plaines, IL, USA).
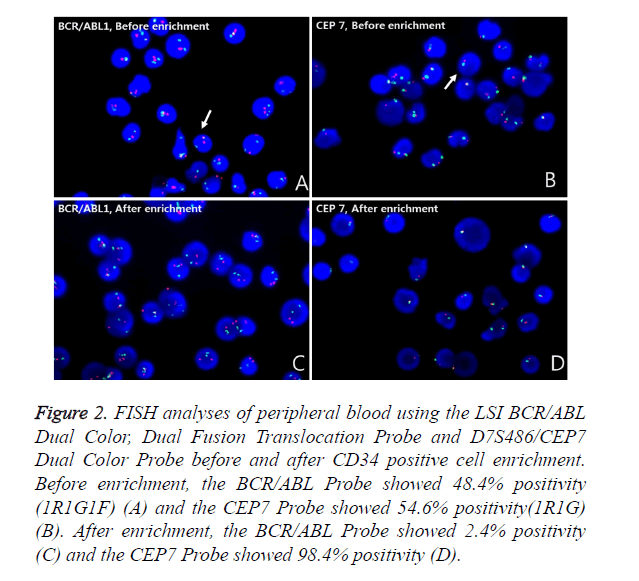
Metaphase FISH showed two different clones; a clone with the BCR/ABL1 rearrangement but without monosomy 7 and a clone with monosomy 7 but without the BCR/ABL1 rearrangement (Figures 1E and 1F). The BCR/ABL1 rearrangement was positive in 30% of the BM cells and 48.4% of the PB cells, with one red, one green and one fusion pattern by FISH (cutoff: 0.6% for fusion). Monosomy 7 was positive in 63.2% of the BM cells and 54.6% of the PB cells, with one red and one green pattern (cutoff: 2.0% for deletion). To determine whether the blasts were a Philadelphia-positive clone or a monosomy 7-positive clone, FISH analysis after CD34 cell enrichment in PB using a commercial kit (Rosette Sep, STEMCELL Technologies, Vancouver, BC, Canada), which greatly enriches CD34-positive cells by creating crosslinks to unwanted cells in human WBCs in whole blood (Figure 2), was performed. Among these enriched cells, the BCR/ABL1 rearrangement was positive in 2.4% and monosomy 7 was positive in 98.4% of cells scored. Thus, we concluded that most of the blasts had monosomy 7. The patient refused all treatment, discharged herself, and was lost to follow-up.
Figure 2. FISH analyses of peripheral blood using the LSI BCR/ABL Dual Color, Dual Fusion Translocation Probe and D7S486/CEP7 Dual Color Probe before and after CD34 positive cell enrichment. Before enrichment, the BCR/ABL Probe showed 48.4% positivity (1R1G1F) (A) and the CEP7 Probe showed 54.6% positivity(1R1G) (B). After enrichment, the BCR/ABL Probe showed 2.4% positivity (C) and the CEP7 Probe showed 98.4% positivity (D).
Discussion
When blast numbers are increased and both a Philadelphia chromosome-positive clone and a clone negative for Philadelphia but with other cytogenetic abnormalities are observed, such cases could be reported as CML with additional cytogenetic abnormalities in a Philadelphia-negative clone or acute leukemia with a Philadelphia-chromosome-positive subclone, according to the history [2]. The emergence of clonal karyotypic abnormalities has been described in Philadelphianegative (Ph–) cells of CML patients treated with chemotherapy, interferon (IFN), and imatinib therapy. The incidences are vary with studies (2% ~17%) [5-7]. Although these cases showed similar prognoses to those of CML patients without cytogenetic abnormalities in Ph– clones, they can, in rare cases, progress to MDS or acute leukemia. Among various accompanying chromosomal abnormalities, monosomy 7 or 7q deletion is associated more frequently with development of MDS or AML [8,9]. However, in these progressed cases, the clinical outcome is variable from resolution with imatinib to death [10]. In rare AML cases, Philadelphia-chromosomepositive subclones can appear during treatment [11]. In this study, blast numbers were clearly increased in the Philadelphia-chromosome-negative clone and the BCR/ABL1 rearrangement was positive in CD34-negative cells. The findings suggest that this could be a case of AML in the setting of CML. A number of mechanisms have been suggested for cytogenetically abnormal Philadelphia chromosome-negative clones in CML [3,12,13]. There might be a mutagenic effect of imatinib. It is also possible that the proliferative stress placed on a small pool of Ph– cells after eradication of Ph+ cells by imatinib results in mutation and the emergence of clonal Ph– hematopoiesis. In addition, the presence of a majority of Ph+ cells at presentation of CML can mask the presence of concomitant Ph– clonal hematopoiesis. However, there is little evidence of the existence of cytogenetically abnormal Ph– clones in pre-treatment CML samples. Although a complete history was not available, this case could support the theories of preexistence. In this case, we were aware of the presence of the additional clone because of monosomy 7 and inversion 3. However, a large portion of acute leukemias show normal karyotypes and if these cases have Philadelphia chromosomes in mature cells, blasts could be mistakenly thought to have the Philadelphia chromosome. Chronic myelogenous leukemia blast crisis and acute leukemia with the Philadelphia chromosome are usually considered difficult to distinguish if a history is not available. Additionally, when only some of the cells are Philadelphia chromosome positive, this case shows the necessity of studies of the cytogenetic characteristics of blasts to better understand the nature of the disease.
Acknowledgement
This work was supported by the Soonchunhyang University Research Fund.
References
- Turhan AG. Chronic myelogenous leukaemia (CML). Atlas Genet Cytogenet Oncol Haematol. 2009; 13: 580-586.
- Swerdlow SH, Campo E (eds): Who classification of tumors of haematopoietic and lymphoid tissue. 4 edn International Agency for Research on Cancer Lyon 2008.
- Lin Y, Bruyère H, Horsman DE, Pantzar T, Barnett MJ, Hogge DE, Nevill TJ, Nantel SH, Sutherland HJ, Toze CL, Shepherd JD, Lavoie JC, Song KW, Smith CA, Forrest DL. Philadelphia-negative clonal hematopoiesis following imatinib therapy in patients with chronic myeloid leukemia: a report of nine cases and analysis of predictive factors. Cancer genetics and cytogenetics 2006; 170: 16-23.
- Terre C, Eclache V, Rousselot P, Imbert M, Charrin C, Gervais C, Mozziconacci MJ, Maarek O, Mossafa H, Auger N, Dastugue N, Talmant P, Van den Akker J, Leonard C, N'Guyen Khac F, Mugneret F, Viguié F, Lafage-Pochitaloff M, Bastie JN, Roux GL, Nicolini F, Maloisel F, Vey N, Laurent G, Recher C, Vigier M, Yacouben Y, Giraudier S, Vernant JP, Salles B, Roussi J, Castaigne S, Leymarie V, Flandrin G, Lessard M. France Intergroupe pour la Leucemie Myeloide Chronique. Report of 34 patients with clonal chromosomal abnormalities in Philadelphia-negative cells during imatinib treatment of Philadelphia-positive chronic myeloid leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K 2004; 18: 1340-1346.
- Fayad L, Kantarjian H, O'Brien S, Seong D, Albitar M, Keating M, Talpaz M. Emergence of new clonal abnormalities following interferon-alpha induced complete cytogenetic response in patients with chronic myeloid leukemia: report of three cases. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K 1997; 11: 767-771.
- Manley R, Cochrane J, McDonald M, Rigby S, Moore A, Kirk A, Clarke S, Crossen PE, Morris CM, Patton WN. Clonally unrelated BCR-ABL-negative acute myeloblastic leukemia masquerading as blast crisis after busulphan and interferon therapy for BCR-ABL-positive chronic myeloid leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K 1999; 13: 126-129.
- Ohtsuka E, Kikuchi H, Abe Y, Moriyama K, Ohno E, Hirota K, Tezono K, Nasu M. Acute myeloblastic leukaemia without Philadelphia chromosome developing after interferon therapy for chronic myelocytic leukaemia with Philadelphia chromosome. British journal of haematology 1995; 90: 951-953.
- Hackanson B, Ruckert A, Lubbert M. Hyperleukocytotic secondary acute myeloid leukemia (AML) with sole monosomy 7 as sequela of Philadelphia-chromosome positive chronic myeloid leukemia (CML). European journal of haematology 2009; 83: 611-612.
- Karimata K, Masuko M, Ushiki T, Kozakai T, Shibasaki Y, Yano T, Abe T, Moriyama M, Toba K, Furukawa T, Aizawa Y. Myelodysplastic syndrome with Ph negative monosomy 7 chromosome following transient bone marrow dysplasia during imatinib treatment for chronic myeloid leukemia. Internal medicine 2011; 50: 481-485.
- Navarro JT, Feliu E, Grau J, Espinet B, Colomer D, Ribera JM, Oriol A, Granada I, Juncà J, Millá F. Monosomy 7 with severe myelodysplasia developing during imatinib treatment of Philadelphia-positive chronic myeloid leukemia: two cases with a different outcome. American journal of hematology 2007; 82: 849-851.
- Neuendorff NR, Schwarz M, Hemmati P, Türkmen S, Bommer C, Burmeister T, Dörken B, le Coutre P, Arnold R, Westermann J. BCR-ABL1(+) acute myeloid leukemia: clonal selection of a BCR-ABL1(-) subclone as a cause of refractory disease with nilotinib treatment. Acta Haematol. 2015; 133: 237-41.
- Bumm T, Müller C, Al-Ali HK, Krohn K, Shepherd P, Schmidt E, Leiblein S, Franke C, Hennig E, Friedrich T, Krahl R, Niederwieser D, Deininger MW. Emergence of clonal cytogenetic abnormalities in Ph- cells in some CML patients in cytogenetic remission to imatinib but restoration of polyclonal hematopoiesis in the majority. Blood 2003; 101: 1941-1949.
- Fialkow PJ, Martin PJ, Najfeld V, Penfold GK, Jacobson RJ, Hansen JA. Evidence for a multistep pathogenesis of chronic myelogenous leukemia. Blood 1981; 58: 158-163.