ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 15
Cynomorium songaricum extract promotes osteoblast differentiation and inhibits lipopolysaccharide induced apoptosis of osteoblast
Wei Xue1#, Nina Dai2#, Fei Feng1, Fei Wang3, Linli Luo4, Jiangrong Huang5* and Zhonghua Cheng1*
1Department of Orthopaedics, Huanggang Central Hospital, Huanggang, Hubei, PR China
2Department of Ultrasound, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, PR China
3Department of Auxiliary, Huanggang Central Hospital, Huanggang, Hubei, PR China
4Department of Nursing, Huanggang Central Hospital, Huanggang, Hubei, PR China
5Department of Integrative Medicine, Medical School of Yangtze University, Jingzhou, Hubei, PR China
#These authors contributed equally to this work
- *Corresponding Authors:
- Jiangrong Huang
Department of Integrative Medicine
Medical School of Yangtze University, PR China
- Zhonghua Cheng
Department of Orthopaedics
Huanggang Central Hospital
PR China
Accepted on May 29, 2017
Bone development is a dynamic process involving the balance between osteoblasts formation and osteoblasts resorption. Lipopolysaccharide (LPS) can break this balance and cause bone injury. Cynomorium songaricum is a Chinese herb that could stimulate osteoblast proliferation and inhibit osteoclast formation. This study aimed to investigate the effects of CSE on the apoptosis and differentiation of osteoblast modulated by LPS. Osteoblastic MC3T3-E1 cell line was used as the experimental model and our results showed that CSE relieved LPS induced inhibition of MC3T3-E1 cell proliferation. Moreover, we found that alkaline phosphatase activity was markedly inhibited by LPS, but could be recovered by CSE. These data suggest that LPS inhibits osteoblast proliferation and differentiation, and CSE antagonizes these inhibitory effects of LPS on osteoblast proliferation and differentiation. Furthermore, we demonstrated that CSE inhibited LPS induced caspase-3 activity in MC3T3-E1 cells, and LPS induced upregulation of caspase-3 and Bax and downregulation of Bcl-2 in MC3T3-E1 cells. These data suggest that LPS induces osteoblast apoptosis while CSE protects osteoblast from LPS induced apoptosis. In conclusion, CSE promotes osteoblast differentiation and inhibits lipopolysaccharide induced apoptosis of osteoblast.
Keywords
Osteoblasts, Cynomorium songaricum, Lipopolysaccharide.
Introduction
Bone development is a dynamic process that involves the balance between osteoblasts formation and osteoblasts resorption [1]. Disruption of this balance would contribute to rheumatoid arthritis, osteomyelitis, bacterial arthritis and infection of orthopedic implants [2]. Lipopolysaccharide (LPS) is a component of the outer membrane of gram-negative bacteria and regulates immune and inflammatory responses [3]. LPS has the capacity to induce bone resorption in vitro and stimulates osteoblasts to secrete osteolytic factors [4]. Thus LPS could break the balance between osteoblasts formation and osteoblasts resorption and cause bone injury.
Cynomorium songaricum (CS) is a well-known root-parasitic plant and is distributed mainly in northwest China [5]. Cynomorium songaricum extract (CSE) has been widely used as Chinese herbal for the treatment of sexual dysfunction, renal disease, and lumbar weakness [6]. Moreover, CSE was shown to promote cell proliferation and neuroblast differentiation in the dentate gyrus of mice by reducing serum corticosterone levels and increasing BDNF levels [7]. CSE also stimulated osteoblast proliferation and inhibited osteoclast formation [8].
In this study, we aimed to investigate the effect of CSE on the apoptosis and differentiation of osteoblastic cell line MC3T3- E1 stimulated with LPS.
Materials and Methods
Preparation of CSE
Cynomorium songaricum Rupr. was purchased from Kyungdong market (Seoul, South Korea). The plants were authenticated by two oriental medical doctors and the voucher specimen was deposited in our laboratory (deposition number: 2012-028). The plants (100 g) were chopped and blended using a blender and soaked in 2 L of 80% ethanol and refluxed at 20°C for 2 h three times. The insoluble materials were removed through centrifugation at 10,000 Xg for 30 min, and the resulting supernatant was concentrated and freeze-dried to yield a powder (yield: 13.2%). Before each experiment, the dried extract was dissolved in distilled and deionized water [9].
Cell culture
Osteoblastic cell line MC3T3-E1 was obtained from ATCC and grown in DMEM supplemented with 10% (v/v) Fetal Bovine Serum (FBS) and 1% (v/v) penicillin-streptomycin solution at 37°C in 5% CO2 humidified air. To stimulate differentiation, MC3T3-E1 cells at a density of 5 × 104 cells/cm2 were cultured in osteogenic differentiation medium (DMEM with 10% FBS, 10 mM HEPES, 50 μg/ml L-ascorbic acid and 5 mM β-GP) for 2 d. On differentiation day 3, the cells were pretreated with or without CSE for 2 h and then treated with or without LPS for indicated period.
MTT assay
The cell growth was measured by MTT assay. Cells were seeded at a density of 5 × 103 cells/well in 96-well plates and cultured for indicated period. At each time interval, the medium was replaced with fresh cell culture medium containing 0.5 mg/mL MTT and incubated for 4 h. Then 150 μl DMSO were added into each well and shaken for 10 min until the crystal was fully dissolved, the plates were measured at 490 nm by a Multiskan MS ELSA reader (xMark Microplate Absorbance Spectrophotometer, BioRad, USA). The relative cell number was normalized by the absorbance from the control cells.
ALP and caspase-3 activity assay
MC3T3-E1 cells were seeded at 5 × 105 cells/cm2 in 100 mm culture dishes and cultured in osteogenic differentiation medium for 2 d. On differentiation d 3, following pretreatment with 0, 25 and 50 μM CSE for 2 h, the cells were treated with 0, 100 and 1,000 ng/ml LPS for 1 and 2 d. The cells were detached aided by a cell scraper in Phosphate-Buffered Saline (PBS), and then centrifuged at 16,000 Xg for 5 min. Cell pellets were placed in 300 μl of lysis buffer (Cytobuster protein extraction reagent; Novagen, Darmstadt, Germany) supplemented with protease inhibitor cocktail. ALP activity in the lysates was determined by the measurement of pnitrophenyl phosphate (pNPP) using a commercial kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s instructions. Relative ALP activity was defined as micromoles of pNPP hydrolyzed per min per 1 g of total protein.
Caspase-3 activity in the lysates was determined by using caspase-3 activity assay kit (Roche) according to the manufacturer’s instructions. Relative caspase-3 activity was defined as micromoles of caspase-3-specific substrate cleaved per min per 1 g of total protein.
Western blot analysis
The total proteins were extracted from the cells and subjected to Western blot analysis following the standard protocol. The primary antibodies used were: Bax (1:1500, Santa Cruz, sc-70408), Bcl-2 (1:1500, Santa Cruz, sc-7382), caspase 3 (1:1000, Santa Cruz, sc-70498), and β-actin (1:1000, Santa Cruz, sc-47778). Horseradish Peroxidase (HRP) labeled secondary antibodies were applied and Enhanced Chemiluminescence (ECL) kit (Pierce, Rockford, IL, USA). was used according to the manufacturer’s instructions. The integrated density mean grey value of the band was analyzed using ImageJ software.
Statistical analysis
Dates are expressed as means ± SE. The differences of means between multiple groups was assessed with the two tailed Student’s t-test (for 2 groups) and the analysis of variance (ANOVA, for >2 groups). P<0.05 was considered statistically significant.
Results
CSE relieves the inhibition of MC3T3-E1 cell proliferation by LPS
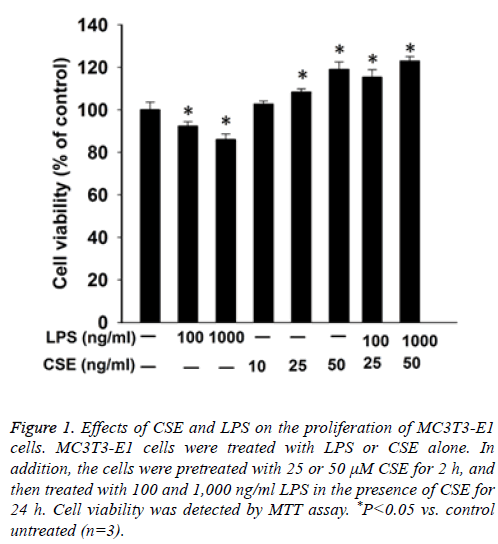
First we performed MTT assay to examine the proliferation of MC3T3-E1 cells. The results showed that LPS inhibited the proliferation of MC3T3-E1 cells in a dose dependent manner. In contrast, CSE increased the proliferation of MC3T3-E1 cells in a dose dependent manner. Furthermore, pretreatment with CSE could relieve the inhibition of the proliferation of MC3T3-E1 cells by LPS in a dose dependent manner (Figure 1).
Figure 1: Effects of CSE and LPS on the proliferation of MC3T3-E1 cells. MC3T3-E1 cells were treated with LPS or CSE alone. In addition, the cells were pretreated with 25 or 50 μM CSE for 2 h, and then treated with 100 and 1,000 ng/ml LPS in the presence of CSE for 24 h. Cell viability was detected by MTT assay. *P<0.05 vs. control untreated (n=3).
CSE promoted LPS induced ALP activity in MC3T3- E1 cells
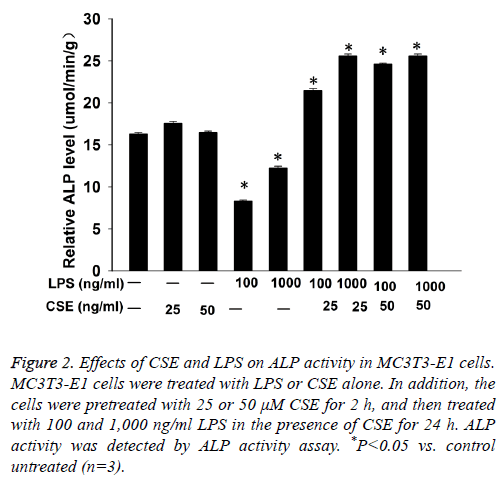
Next we performed ALP activity assay to examine the differentiation of MC3T3-E1 cells. The results showed that LPS inhibited ALP activity in MC3T3-E1 cells in a dose dependent manner. CSE had no significant effect on ALP activity in MC3T3-E1 cells, but pretreatment with CSE could significantly increase LPS induced ALP activity in MC3T3-E1 cells (Figure 2).
Figure 2: Effects of CSE and LPS on ALP activity in MC3T3-E1 cells. MC3T3-E1 cells were treated with LPS or CSE alone. In addition, the cells were pretreated with 25 or 50 μM CSE for 2 h, and then treated with 100 and 1,000 ng/ml LPS in the presence of CSE for 24 h. ALP activity was detected by ALP activity assay. *P<0.05 vs. control untreated (n=3).
CSE inhibited LPS induced caspase-3 activity in MC3T3-E1 cells
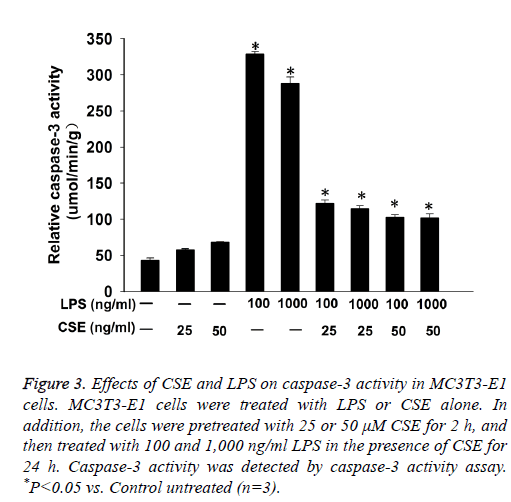
Caspase-3 activity assay showed that LPS induced caspase-3 activity in MC3T3-E1 cells. CSE had no significant effect on caspase-3 activity in MC3T3-E1 cells, but pretreatment with CSE could significantly decrease LPS induced caspase-3 activity in MC3T3-E1 cells (Figure 3).
Figure 3: Effects of CSE and LPS on caspase-3 activity in MC3T3-E1 cells. MC3T3-E1 cells were treated with LPS or CSE alone. In addition, the cells were pretreated with 25 or 50 μM CSE for 2 h, and then treated with 100 and 1,000 ng/ml LPS in the presence of CSE for 24 h. Caspase-3 activity was detected by caspase-3 activity assay. *P<0.05 vs. Control untreated (n=3).
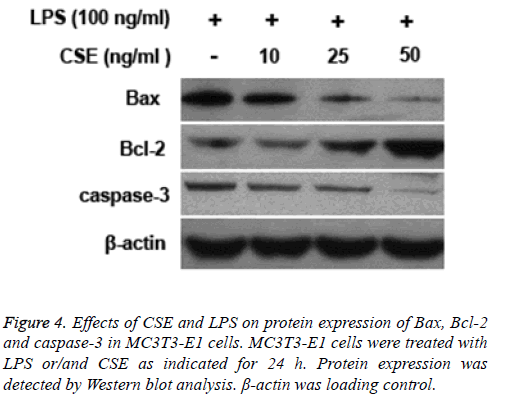
CSE modulated protein expression of Bax, Bcl-2 and caspase-3 in MC3T3-E1 cells
LPS treatment led to increased protein expression levels of caspase-3 and Bax and decreased expression of Bcl-2 in MC3T3-E1 cells. However, pretreatment with CSE could inhibit LPS induced upregulation of caspase-3 and Bax and downregulation of Bcl-2 in MC3T3-E1 cells (Figure 4).
Discussion
Bone injury is hard to cure because of bacteria induced activation of inflammatory responses [10]. LPS is a component of cell membrane of gram-negative bacteria and could break the balance between osteoblasts formation and osteoblasts resorption [11]. Cynomorium songaricum is a Chinese herb which has been used for the treatment of osteoporosis [12]. In this study, we found that LPS significantly inhibited MC3T3- E1 cell proliferation, consistent with previous study [13]. However, CSE relieved LPS induced inhibition of MC3T3-E1 cell proliferation. ALP is an essential marker in osteoblast differentiation [14]. We found that ALP activity was markedly inhibited by LPS, but could be recovered by CSE. These data suggest that LPS inhibits osteoblast proliferation and differentiation, and CSE antagonizes these inhibitory effects of LPS on osteoblast proliferation and differentiation.
LPS can induce cell apoptosis via extrinsic pathway, and this may contribute to bacteria induced bone destruction [15]. We examined the effect of CSE on the apoptosis of MC3T3-E1 cells stimulated by LPS. The results showed that caspase-3 activity was significantly increased in MC3T3-E1 cells treated with LPS, but CSE inhibited LPS induced caspase-3 activity in MC3T3-E1 cells. Moreover, LPS treatment led to the upregulation of pro-apoptotic factors such as caspase-3 and Bax and the downregulation of anti-apoptotic factor Bcl-2 in MC3T3-E1 cells. However, CSE could decrease the expression of caspase-3 and Bax and increase the expression of Bcl-2 in MC3T3-E1 cells. These data suggest that LPS induces osteoblast apoptosis while CSE protects osteoblast from LPS induced apoptosis.
In conclusion, our study suggests that CSE promotes osteoblast differentiation and inhibits lipopolysaccharide induced apoptosis of osteoblast. CSE could be used to treat bacteria induced bone injury.
Conflict of Interest
None declared.
Acknowledgement
This study was supported by basic disciplines of research and development fund of the department of science and technology (No. 2014JCY004) and scientific research foundation for talented scholars of the personnel department of Yangtze University (No. 801250010132).
References
- Bae HW, Patel VV, Sardar ZM. Transient local bone remodeling effects of rhbmp-2 in an ovine interbody spine fusion model. J Bone Joint Surg Am 2016; 98: 2061-2070.
- Azeena S, Subhapradha N, Selvamurugan N. Antibacterial activity of agricultural waste derived wollastonite doped with copper for bone tissue engineering. Mater Sci Eng C Mater Biol Appl 2017; 71: 1156-1165.
- Teramachi J, Inagaki Y, Shinohara H. PKR regulates LPS-induced osteoclast formation and bone destruction in vitro and in vivo. Oral Dis 2016.
- Zhang Y, Yan M, Yu QF. Puerarin prevents LPS-induced osteoclast formation and bone loss via inhibition of AKT activation. Biol Pharm Bull 2016; 39: 2028-2035.
- Tuvaanjav S, Shuqin H, Komata M. Isolation and antiviral activity of water-soluble Cynomorium songaricum Rupr. polysaccharides. J Asian Nat Prod Res 2016; 18: 159-171.
- Liu HP, Chang RF, Wu YS. The Yang-Tonifying herbal medicine Cynomorium songaricum extends lifespan and delays aging in drosophila. Evid Based Complement Alternat Med 2012; 2012: 735481.
- Yoo DY, Choi JH, Kim W. Cynomorium songaricum extract enhances novel object recognition, cell proliferation and neuroblast differentiation in the mice via improving hippocampal environment. BMC Complement Altern Med 2014; 14: 5.
- Yin J, Tezuka Y, Kouda K. Antiosteoporotic activity of the water extract of Dioscorea spongiosa. Biol Pharm Bull 2004; 27: 583-586.
- Yoo DY, Choi JH, Kim W. Cynomorium songaricum extract enhances novel object recognition, cell proliferation and neuroblast differentiation in the mice via improving hippocampal environment. BMC Complement Altern Med 2014; 14: 5.
- Lapérine O, Cloitre A. Interleukin-33 and RANK-L Interplay in the Alveolar Bone Loss Associated to Periodontitis. PLoS One 2016; 11: e0168080.
- Kanno Y, Ishisaki A, Miyashita M. The blocking of uPAR suppresses lipopolysaccharide-induced inflammatory osteoclastogenesis and the resultant bone loss through attenuation of integrin ß3/Akt pathway. Immun Inflamm Dis 2016; 4: 338-349.
- Cui Z, Guo Z, Miao J, Wang Z, Li Q. The genus Cynomorium in China: an ethnopharmacological and phytochemical review. J Ethnopharmacol 2013; 147: 1-15.
- Liu YH, Huang D, Li ZJ. Toll-like receptor-4-dependence of the lipopolysaccharide-mediated inhibition of osteoblast differentiation. Genet Mol Res 2016; 15.
- Lin FX, Du SX, Liu DZ. Naringin promotes osteogenic differentiation of bone marrow stromal cells by up-regulating Foxc2 expression via the IHH signaling pathway. Am J Transl Res 2016; 8: 5098-5107.
- Fragoso IT, Ribeiro EL, Gomes FO. Diethylcarbamazine attenuates LPS-induced acute lung injury in mice by apoptosis of inflammatory cells. Pharmacol Rep 2016; 69: 81-89.