ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 9
Comparative survival and safety analysis of cisplatin based regimens in gall bladder cancer patients: A retrospective cohort study
1Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research, Hajipur, India
2Department of Oncology, Mahavir Cancer Sansthan, Patna, India
3Department of Clinical Medicine, Rajendra Memorial Research Institute of Medical Sciences, Agamkuan, Patna, India
4Department of Molecular Biology, Rajendra Memorial Research Institute of Medical Sciences, Agamkuan, Patna, India
#These authors contributed equally to this work
- *Corresponding Author:
- Krishna Pandey
Department of Clinical Medicine
Rajendra Memorial Research Institute of Medical Sciences, India
Accepted on February 17, 2017
Aim: This is a retrospective cohort study of patients who were treated with different regimens of adjuvant chemotherapy after cholecystectomy due to Gall Bladder Cancer (GBC).
Patients and Methods: Data of GBC Patients registered between January 2011 to January 2015 at Mahavir Cancer Sansthan treated with 5-FU plus Cisplatin and Gemcitabine plus Cisplatin as adjuvant chemotherapy were reviewed retrospectively. Eighty patients were enrolled and data was analysed for overall survival and progression free survival. Causality, preventability and severity of Adverse Drug Reactions (ADRs) were also evaluated.
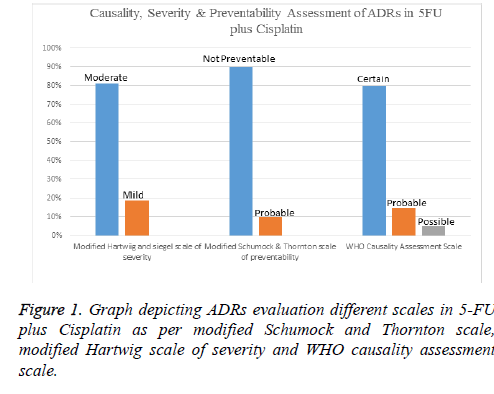
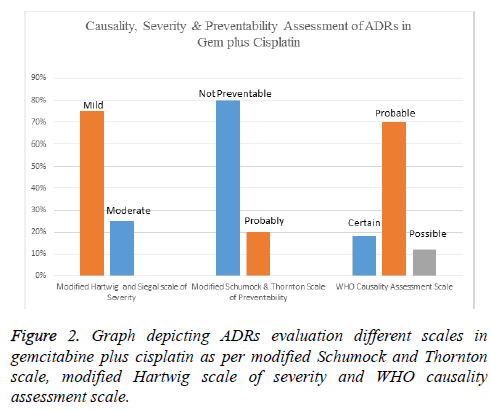
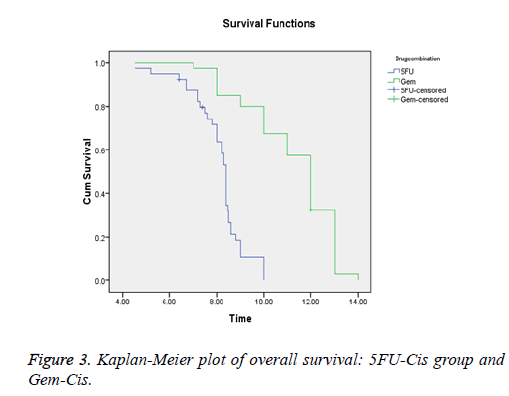
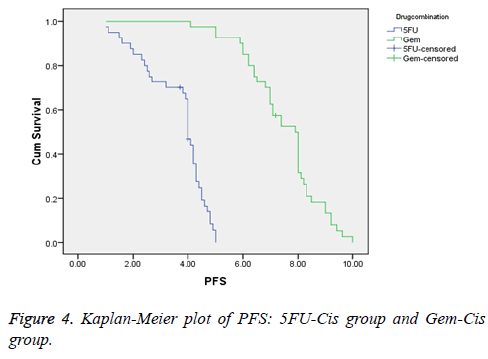
Results: Forty patients were assigned with 5FU-Cis and Gem-Cis each. 5FU-Cis gave more ADRs than Gem-Cis. Incidence of ADRs in 5FU-Cis regimen was 62.5% and that of Gem-Cis was 40%. According Modified Hartwig and Siegel scale, “80%” of ADRs shown by 5FU-Cis were moderately severe. Based on Modified Schumock & Thornton Preventability scale, 5FU-Cis and Gem-Cis showed “90%” and “80%” Not Preventable ADRs respectively. As per WHO-UMC Causality assessment scale, “80%” were certain with 5FU-Cis. Gem-Cis showed a mean OS of 11.2 months (95% CI, 10.6-11.8) and mean PFS of 7.4 months (95% CI, 7.3-8.4). 5FU-Cis had a mean OS of 8.1 months (95% CI, 7.7-8.4) and mean PFS of 3.4 months (95% CI, 3.8-4.1).
Conclusion: Direct comparison of 5FU-Cis and Gem-Cis showed the latter regimen was superior in OS and PFS. Further prospective studies which define the efficacy and toxicity of these regimens are recommended.
Keywords
Adjuvant chemotherapy, Fluoropyrimidines, Gemcitabine, Adverse drug reactions, Overall survival, Progression free survival.
Introduction
Gall bladder cancer, one of the most common biliary tract cancers, is a lethal malignancy and its incidence varies in accordance to various ethnic groups and geographical conditions [1,2]. In India, north and north eastern states like Uttar Pradesh, Bihar, Orissa, West Bengal and Assam has one of the highest reported incidence of Gallbladder Cancer (GBC) in the world [3,4]. Gall stones are considered as one of the most important risk factor for GBC followed by obesity [5]. Presence of high arsenic content in the Gangetic-Zone is another risk factor [6]. This study was conducted in Bihar which is one of the arsenic hot spots in India. Districts of Bihar which are close to Gangetic-Zone may contribute more GBC patients. Other risk factors include porcelain gall bladder (gall bladder covered with calcium deposits), older age (after the age of 45 years there is an increase in incidence of GBC and reaches maximum at the age of 65 years), gall bladder polyps [7] primary sclerosing cholangitis [8], typhoid disease and family history [1]. Women are two times more vulnerable than men and in north Indian cities; GBC is the leading digestive cancer in women [1].
GBC is usually found in its advanced stage as the symptoms are indistinct and are comparable to diseases like cholelithiasis and cholecystitis. One out of five gall bladder cancers are detected at early stages. Patients with advanced GBC have a mean survival rate of 6 months [1]. In advanced/unresectable GBC, cancer cells have invaded distant tissues or they are in such a place where it is too difficult to be removed entirely by surgery [5]. In resectable GBC, surgery is done and adjuvant chemotherapy is given. The main drugs used in the treatment of biliary tract cancer including GBC are Fluoropyrimidines (5-FU), Gemcitabine and Platinum compounds [9]. 5FU plus cisplatin combination is preferred as first line drug by patients in developing countries like India because of their low price compared to other newly introduced drug regimens.
As we have very limited data in the adjuvant chemotherapy of GBC, it has become customary to extrapolate the data available from the studies of neo-adjuvant chemotherapy in advanced GBC and other advanced biliary tract cancers. Here in this study, we are analysing the outcome of 5-FU plus Cisplatin and Gemcitabine plus Cisplatin combination regimens when they are given as adjuvant chemotherapy. The National Comprehensive Cancer Network guidelines suggest fluoropyrimidine or Gemcitabine chemotherapy in patients with>T1N0 gallbladder cancer following curative surgery [10]. So this study was initiated with such an intention to provide the outcome of these drug regimens exclusively on GBC patients. More than that, we lack the data of effect of these regimens on Indian population with GBC.
Patients and Methods
This study was conducted at Mahavir Cancer Sansthan, Patna, India and was approved by Mahavir Institutional Ethics Committee. The retrospective data of confirmed GBC patients were collected during the period January 2011 to January 2015.
Male and female patients, >18 years of age, had biopsy or fine needle aspiration cytology–proven GBC and received 5-FU plus Cisplatin or Gemcitabine plus Cisplatin as first line adjuvant chemotherapy were included in the study.
Patients who didn’t complete prescribed chemotherapy cycles or received neo-adjuvant chemotherapy/chemo-radiation as adjuvant therapy were excluded from the study. Patients with co-morbid conditions such as diabetes mellitus, cardiovascular diseases and psychological problems were also excluded from the study. Only the data of eligible patients were considered for evaluation.
In 5-FU plus Cisplatin combination, Inj. 5FU 1000 mg/m2 and Cisplatin 50 mg/m2 were administered on day 1 and 2 of each cycle. In the Gemcitabine plus Cisplatin regimen, each cycle comprised cisplatin 25 mg/m2 followed by gemcitabine 1000 mg/m2, each administered on days 1 and 8 every 3 weeks. Patients received treatment cycles at an interval of 21 days provided that he/she has recovered from any drug related toxicity associated with the previous one. Adverse Drug Reactions (ADRs) of 5-FU plus Cisplatin and Gemcitabine plus Cisplatin were assessed by different standardized scales. The severity of ADR was assessed by Modified Hartwig and Siegel scale [11]. The reported ADRs were categorized into mild, moderate and severe. Based on Modified Schumock and Thornton scale, ADRs were categorized into definitely preventable, probably preventable and not preventable [12].
Causality of ADRs was assessed using WHO-UMC Causality assessment scale [13].
Statistical analysis
Results were expressed as percentage after the analysis of data using descriptive statistics. P value<0.05 were considered statistically significant. Data was analysed using SPSS v 16. Overall Survival (OS) and Progression Free Survival (PFS) were calculated using Kaplan-Meier method and compared against log rank test.
Results
Patient’s characteristics
The data of 105 patients were screened for this study and 80 patients’ data were found to be eligible for the analysis. Out of eighty patients, forty patients were assigned in each of the drug regimen. Table 1 represents the baseline characteristics of patients included. In both arms most of the patients were females, 87.5% in 5-FU-C is and 95% in Gem-Cis, respectively. Majority of the patients in both arms were in normal Body Mass Index (BMI). As we are studying the effect of drug after surgery, most of the patients were in stage III (30%) and stage II (51%). Least in stage I (19%). Surgery is not a treatment option in Stage IV (NCCN Guidelines Version 1.2016). Most of the patients were from Patna district (26%) followed by Siwan (18.75%) of Bihar, India.
| Variable | 5FU plus cisplatin | Gemcitabine plus cisplatin | P value |
|---|---|---|---|
| Age in years | N=40 | N=40 | |
| Median | 0.12 | ||
| Mean | 50 | 52 | |
| Range | 49.3±8.9 | 52.64±8.2 | |
| Gender-n (%) | 34-76 | 41-75 | 0.23 |
| Male | |||
| Female | 05 (12.5) | 02 (5) | |
| BMI | 35 (87.5) | 38 (95) | 0.12 |
| Underweight | 16 (40) | 09 (22.5) | |
| Normal weight | 19 (47.5) | 28 (70) | |
| Overweight | 5 (12.5) | 3 (7.5) | |
| Obese | 0 | 0 |
Table 1. Baseline characteristics of participants in study.
Evaluation of safety revealed that there exists an association between drug regimen and ADRs (statistically significant P=0.04). Both the drug regimens had their devastating effect on the haematological system with Gem-Cis regimen being a little innocuous (50%). Anaemia and thrombocytopenia shown by these two regimens are statistically significant. 5-FU-Cis regimen is hegemonic in all the evaluated systems of body. All the details are mentioned in Table 2. Incidence of ADRs in 5- FU-C is regimen was 62.5% and that of Gem-Cis was 40% (Table 3). Severity measurement of regimens indicated that majority of ADRs reported by 5-FU-Cis were severe (80%). Modified Schumock and Thornton scale reported that “Not Preventable” ADRs were dominantly shown by both of these regimens. According to WHO-UMC Causality assessment scale, 80% of the ADRs were “Certain” in 5-FU-Cis regimen but only Probable ADRs were reported with “Gem-Cis”. Figures 1 and 2 depict the above mentioned data.
| Organ system involved | Adverse Drug Reactions (ADRs) | 5FU plus cisplatin (N=40) | Gemcitabine plus cisplatin (N=40) | P value |
|---|---|---|---|---|
| Blood | Anaemia | 14 (36.66) | 04 (10) | 0.007 |
| Leucopenia | 11 (27.5) | 05 (12.5) | 0.09 | |
| Neutropenia | 02 (5) | 01 (2.5) | 0.74 | |
| Thrombocytopenia | 05 (12.5) | 13 (32.5) | 0.05 | |
| Gastrointestinal System | Vomiting | 08 (20) | 05 (12.5) | 0.32 |
| Constipation | 05 (12.5) | 04 (10) | 0.57 | |
| Gastritis | 02 (5) | 05 (12.5) | 0.11 | |
| Oral ulcer | 01 (2.5) | 0 | 0.31 | |
| Dysphagia | 0 (2.5) | 0 | 0.55 | |
| Musculoskeletal System | Weakness | 09 (22.5) | 05 (12.5) | 0.15 |
| Respiratory System | S.O.B | 03 (7.5) | 02 (5) | 0.24 |
| Cough | 01 (2.5) | 01 (2.5) | 1 | |
| CNS &PNS | Peripheral Neuropathy | 02 (5) | 01 (2.5) | 0.55 |
| Fever | 02 (5) | 04 (10) | 0.14 | |
| Renal System | Dysuria | 01 (2.5) | 0 | 0.31 |
| Skin | Alopecia | 04 (10) | 02 (2.5) | 0.24 |
Table 2. Adverse reactions associated with treatment.
| Variable | Adverse Drug Reactions (ADRs) | P-value | |
|---|---|---|---|
| Yes | No | ||
| Drug combination (n(%)) | 0.04 | ||
| 5FU plus cisplatin | 25 (62.5) | 15 (37.5) | |
| Gemcitabine plus cisplatin | 16 (40) | 24 (60) | |
Table 3. Drug regimen and ADRs.
Efficacy
5FU-Cis regimen showed a median OS of 8.1 months (95% CI, 7.7-8.4). On the other hand, Gem-Cis regimen had a mean OS of 11.2 months (95% CI, 10.6-11.8). Comparative assessment of PFS revealed 3.4 months (95% CI, 3.8-4.1) in 5FU-Cis and 7.4 months (95% CI, 7.3-8.4) in Gem-Cis regimen. P<0.001 in both OS and PFS. Figures 3 and 4 depict the data.
Discussion
5-FU and Gemcitabine are antimetabolites whereas cisplatin belongs to platinum complex. 5-FU inhibits thymidylate synthase and selective failure of DNA synthesis occurs due to non-availability of thymidylate [14]. It is mainly used for the treatment of solid tumors like breast, colon, urinary bladder and liver. It is used topically in cutaneous basal cell carcinoma. It exerts primary toxicity on gastrointestinal system and bone marrow. To control cytotoxic drug induced vomiting, Ondansetron, a 5-HT3 antagonist, is used [15]. Gemcitabine is a pro-drug. Once it is transported into the cell, it is phosphorylated by deoxycytidine kinase to an active form.
Both gemcitabine diphosphate and gemcitabine triphosphate inhibit processes required for DNA synthesis [16]. Cisplatin, on the other hand causes cross linking of DNA. It is effective in metastatic testicular and ovarian carcinoma. It is also widely used in many solid tumors like lung, bladder, gastric hepatic, esophageal, head and neck carcinomas. Cisplatin is one of the highly emetic drugs and its most important toxicity is renal impairment. Antiemetics are priorly administered before infusing it. Amifostine, which is an organic thiophosphate, is specifically used for the prophylaxis of Cisplatin induced nephrotoxicity [17]. This study is done exclusively on GBC patients and the results from this study may help the physicians while choosing treatment regimens for their patients. 5-FU plus Cisplatin is economical compared to other newly introduced drug combinations like Gemcitabine plus Cisplatin and Gemcitabine plus Oxaliplatin. This study shall help the physicians to weigh the pros and cons of regimen and take the best step forward.
In our study, majority of the patients were females. So this study supports female gender as a risk factor for gall bladder cancer which is mentioned in the National Cancer Register Programme [18]. Maximum patients were from Patna followed by Siwan, India which strengthens that there is high prevalence of GBC in the population along the Gangetic basin and high arsenic concentration in drinking water may be a cause [19,20]. In this study, obesity contrasts as a risk factor as most of the patients had normal BMI.
As far as risk is concerned, maximum ADRs were reported in the haematological system followed by gastrointestinal system by both of the regimens which is similar to the study conducted by Smita et al. [21]. According to Modified Hartwig and Siegal Scale, most of the ADRs were moderate (81%) and mild (19%) by 5FU-Cis and this is in contrast to the study conducted by Suriendran et al. [22] where moderate ADRs were low, mild ADRs were 80% and no severe ADRs. But Gem-Cis regimen reported more mild ADRs. WHO-UMC and Modified Schumock and Thornton scales assessment in both of the regimen revealed that Gem-Cis regimen showed more “Probable” ADRs and in both of the regimens’ ADRs were in “Not Preventable” section. To our knowledge, it is the first study that compares the Severity, Causality and Preventability of ADRs of two regimens with these scales.
The results of our study found a better efficacy for Gem-Cis regimen in terms of OS and PFS. Median OS for Gem-Cis was 11.2 months which is somewhat different from the study conducted by Adina et al. [9,23] where OS was 9.1 months. The median OS in 5FU-Cis regimen was 10.3 months in the same study but in our study median OS was reported to be 8.1 months. Adina et al. conducted study which was on unresectable/locally advanced GBC. So comparison of efficacy of both the drugs is not exclusively correct. Median OS of Gem-Cis was similar to a study conducted by Valle et al. [24] where it was observed as 11.7 months. Median PFS of both the regimens were different from other studies. PFS for Gem-Cis in the study of Valle et al was 8 months but in our study it was 7.1 months. Valle also conducted study on unresectable GBC patients. There were other studies also comparing these regimens but they were done on unresectable GBC patients [25].
Conclusion
Our results points that Gem-Cis has shown superiority in efficacy and safety to 5FU-Cis regimen. In short, Gem-Cis regimen is an appropriate treatment option for adjuvant chemotherapy in resected GBC patients. A prospective study comparing the efficacy and safety of both these regimens in resected GBC patients is inevitable.
Acknowledgements
This study was supported by grants from the National Institute of Pharmaceutical Education and Research (NIPER), Hajipur under the aegis of Department of Pharmaceutical, Ministry of Chemicals and Fertilizers, Government of India, New Delhi, India.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Consensus Document for the Management of Gall Bladder Cancer: An Outcome of ICMR subcommittee on Gall Bladder Cancer, Indian Council of Medical Research Publication, 2014.
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006; 118: 1591-1602.
- Jagannath P, Dhir V, Mohandas KM. Geographic patterns in incidence of Gall Bladder cancer in India and the possible etiopathological factors. HPB 2000; 2: 168-169.
- Zatonski WA, Lowenfels AB, Boyle P, Maisonneuve P, Bueno de Mesquita HB. Epidemiologic aspects of gallbladder cancer: a case-control study of the Search Program of the International Agency for Research on Cancer. J Natl Cancer Inst 1997; 89: 1132-1138.
- Unisa S, Jagannath P, Dhir V, Khandelwal C, Sarangi L, Roy TK. Population-based study to estimate prevalence and determine risk factors of gallbladder diseases in the rural Gangetic basin of North India. HPB 2011; 13: 117-125.
- Acharyya SK, Chakraborty P, Lahiri S. Arsenic poisoning in the Ganges delta. Nature 1999; 401: 545-546.
- Myers RP, Shaffer EA, Beck PL. Gallbladder polyps: epidemiology, natural history and management. Can J Gastroenterol 2002; 16: 187-194.
- Dhir V, Mohandas KM. Epidemiology of digestive tract cancers in India IV. Gall bladder and pancreas. Indian J Gastroenterol 1999; 18: 24-28.
- Croitoru A, Gramaticu I, Dinu I. Fluoropyrimidines plus cisplatin versus gemcitabine/gemcitabine plus cisplatin in locally advanced and metastatic biliary tract carcinoma-A retrospective study. J Gastrointestin Liver Dis 2012; 21: 277-284.
- Kanthan R, Senger JL, Ahmed S, Kanthan SC. Gallbladder cancer in the 21st century. J Oncol 2015; 2015: 967472.
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992; 49: 2229-2232.
- Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm 1992; 27: 538.
- The use of the WHO-UMC system for standardized case causality assessment http://who-umc.org/Graphics/ 24734 [Internet].
- Katzung GB, Masters BS, Trevor JA. Basic and Clinical Pharmacology. NY McGraw-Hill Medical London McGraw-Hill 2012; 12: 953-958.
- Lee A, Thomas SHL. Adverse drug reactions. Clin Pharm Therap Churchill Livingstone 2003; 3: 33-34.
- Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. SeminOncol 1995; 22: 3-10.
- Tripati KD. Essentials of pharmacology. JaypeePubl (7th edn.) 2013: 170-176.
- National Cancer Registry Programme. Consolidated report of the population based cancer registries 1990-1996. New Delhi: Indian Council of Medical Research, 2001.
- Chakraborti D, Mukherjee SC, Pati S. Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: a future danger? Environ Health Perspect 2003; 111: 1194-1201.
- Chhabra D, Oda K, Jagannath P, Utsunomiya H, Takekoshi S. Chronic heavy metal exposure and gallbladder cancer risk in India, a comparative study with Japan. Asian Pac J Cancer Prev 2012; 13: 187-190.
- Khandelwal S, Bairy KL, Vidyasagar MS, Chogtu B, Sharan K. Adverse drug reaction profile of cancer patients on chemotherapy in a tertiary care hospital. Int J Pharm Bio Sci 2015; 6: 233-244.
- Surendiran A, Balamurugan N, Gunaseelan K, Akhtar S, Reddy KS, Adithan C. Adverse drug reaction profile of cisplatin-based chemotherapy regimen in a tertiary care hospital in India: An evaluative study. Indian J Pharmacol 2010; 42: 40-43.
- Kim MJ, Oh DY, Lee SH, Kim DW, Im SA. Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study. BMC Cancer 2008; 8: 374.
- Valle J, Wasan H, Daniel H. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010; 362: 1273-1281.
- Kim MJ, Oh DY, Lee SH, Kim DW, Im SA. Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study. BMC Cancer 2008; 8: 374.