ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 14
Comparative study of different phytomolecules acting on hRBC to treat rheumatoid arthritis
1TAAB Biostudy Services, Jadavpur, Kolkata, India
2Pharmaceutical Technology, Jadavpur University, India
3College of Pharmacy, Taibah University, Al-Madinah Al-Munawwarah, Kingdom of Saudi Arabia
4Patel College of Pharmacy, Bhopal, Madhya Pradesh, India
5College of Science, Taibah University, Al-Madinah Al-Munawwarah, Kingdom of Saudi Arabia
- *Corresponding Author:
- Mohi Iqbal Mohammed Abdul
College of Pharmacy
Taibah University
Kingdom of Saudi Arabia
Accepted date: July 20, 2018
DOI: 10.4066/biomedicalresearch.29-18-846
Visit for more related articles at Biomedical ResearchRheumatoid arthritis is a chronic inflammatory disease condition in synovial joints causing progressive destruction of synovial fluid resulting moderate to severe pain. Oxidative stress plays a major role in inflammation. Free radicals which are found to have in our body, either as a product of metabolism or incorporated from our environment, plays a major role in formation of oxidative stress, that leads to inflammation. To reduce this oxidative stress anti-oxidant may contribute in management of inflammation. In this study, the antioxidant and anti-inflammatory property of tartaric acid and β- carotene has established and compared with vitamin C and diclofenac sodium as standard drug. The free radical scavenging assay and protein denaturation assay was conducted for this purpose. The % inhibition confirmed the activity of individual molecules. According to the study outcome, Tartaric Acid is more effective antioxidant and anti-inflammatory agent than β-carotene. And so far it can be used as anti-rheumatoid arthritic agent. The IC50 values and graphical representations confirm the result.
Keywords
Antioxidant, Inflammation, Free radicals, Oxidative stress, ROS, RNS.
Introduction
RA is a special type of inflammation in synovial joints, a progressive annihilation of synovial fluid shows frequent inflammatory outcomes soreness, heat, blush, swelling and loss of function. And rigid joints cause mutilation of functional status, and as well symptoms such as exhaustion, low-grade fever and loss of weight [1]. Free radical in body either from the influence of metabolic by-product or from environment source causes serious oxidative stress, which produces ROS and RNS, that is responsible for the pathogenesis of different disease and illness [2-8]. Several available medicines often cause alteration in serum concentration, but persistent and continuous joint inflammation causes progressive destruction of synovial joints. In this context, using phytomolecules having antioxidant would have less side effects and cost effective.
Free radicals interferers with the functional stability of erythrocytes. Oxidative stress in RBCs by available free radicals cause hemolysis, due to high reactivity of the free radicals on the cell membrane causes potential damage of RBCs which decreases tissue oxygenation. During hemolysis, haemoglobin, present inside RBC is released into the blood stream. The released hemoglobin in the external microenvironment is oxidized by molecular oxygen to form methemoglobin [9-12].
Phytomolecules i.e., tartaric acid and β carotene has been selected as anti-oxidants. Tartaric acid is a naturally occurring dicarboxylic acid found in many plants, presence of carboxylic acid moiety in structure of tartaric acid potentiate its antioxidant activity. Literature survey confirms the in-vitro antioxidant activity of tartaric acid [9] from molecular pharmacology aspect; the OH group diminishes the free radical formation among cell, and controls oxidative stress within cell. β-carotene is a carotenoid that is a precursor of vitamin A. Evidence suggests that β-carotene shows anti-oxidant activity. From stereochemistry aspect β-carotene is basically a terpene, the long chain isoprene ring in β-carotene probably the reason for anti-oxidant effect.
In present study the comparative anti-oxidant and antiinflammatory activity of tartaric acid and β carotene, has been tried to establish with standard anti-oxidant vitamin C and standard anti-inflammatory agent as diclofenac sodium. The graphical illustrations discussed to establish the comparison study. The radical scavenging activity was checked to establish the anti-oxidant activity, and protein denaturation assay was performed to establish anti-arthritic activity.
Materials and Methods
Chemicals and reagents and apparatus
Tartaric acid and β-carotene (marketed from SIGMAALDRICH), vitamin-C (imported from Sigma-Aldrich), diclofenac sodium (purchased from local licensed pharmacy).
Essential lab equipment including glass apparatus, Small Bench lab Centrifuge, UV visible spectrophotometer ((V-630), incubator, micro pipettes, syringes and EDTA vials etc.
Collection of blood sample
5 ml of blood from different healthy human volunteers (age between 22-45 y) was collected in EDTA containing vials by veinpuncture method along with their filled up consent form. The blood from vial immediately transferred to 15 ml centrifuge tube. With 5 ml of blood 10 ml of freshly prepared PBS added, and it centrifuged for 5 minutes at 3000 rpm. Then the supernatant was taken off and with the remaining settled RBCs again 10 ml of PBS added and centrifugation was repeated for three times to remove the excess plasma [3] and ultimately the settled RBCs were taken.
Preparation of hemosylate
The previously collected RBCs were mixed with phosphate buffer in 1:20 ratio to prepare hemosylate (The product resulting from the lysis of red blood cells.)
Experimental design
Antioxidant activity: Drug activity against nitrite induced lysis of hRBC was performed [13] to 1 ml of hemosylate, different concentration of drug sample is induced. And 0.01 ml of 6.0 mmol/L sodium nitrite added so that methohemohlobin formed. And the amount of protection to the RBC by the drug was measured in spectrophotometrically at 631 nm after 50 min of incubation at 37°C [3].
% inhibition was calculated by
% inhibition=((abs of control-abs of test)/abs of control) × 100
Drug activity against hydrogen peroxide induced lysis of hRBC was performed accordingly [14] to 1 ml of hemosylate, different concentration of drug sample was induced concomitantly with 0.1 ml of Hydrogen peroxide added. Then all the test tubes were incubated for 1 h and centrifuged for 10 min at 2000 rpm following the separation of supernatant and absorbance was measured at 540 nm. Vitamin C was taken as standard [3]. % inhibition was calculated by,
% inhibition=((abs of control-abs of test)/abs of control) × 100
In vitro anti-inflammatory activity [15,16]: Protein denaturation assay method was followed to evaluate antiinflammatory activity. A stock solution of 0.2% w/v of BSA was prepared in phosphate buffer and pH was adjusted from 8.54 to 6.74 using HCL. Test compounds (β-carrotene and tartaric acid respectively) and standard compound (diclofenac sodium) was prepared.
Few replicates of BSA stock solution with test and standard compound was pipette out and mixed and the final volume of each tube was adjusted with distilled water upto 5 ml. And incubated at 37°C for 30 min, followed by induction of 55°C heat as a thermal stress for 5 min.
Then all the samples are cooled down for 20 min. Absorbance was checked at 660 nm.
Result
Hemoglobin is structurally formed with a heme molecule and globin protein. In structurally analysis it was observed previously that the smallest unit of the heme is the pyrrole moiety which is formed when succinyl-CoA binds with glycine by hydrogen bonding. Four of the pyrrole molecules combine to form porphyrin or protoporphyrin IX [17]. These molecules combine with ferrous iron (Fe++) to form the heme molecule. Each heme molecule combines with a long polypeptide chain known as a globin, forming a hemoglobin chain. Each hemoglobin chain contains an atom of iron capable of binding loosely with one molecule of oxygen; therefore, each hemoglobin molecule containing four hemoglobin chains can transport four oxygen molecules [18].
The main function of haemoglobin in blood cell is to carry oxygen to other cells and tissues. Three types of haemoglobin exist. These three types are commonly known as dyshemoglobins. They are carboxyhemoglobin, sulfhemoglobin, methemoglobin [19].
Methemoglobin (MetHb) is another dysfunctional form of hemoglobin incapable of transporting oxygen. Methemoglobinemia occurs with elevated levels of MetHb. Hemoglobin contains iron in the ferrous state (Fe++) whereas MetHb contains iron in the ferric state (Fe+++). Similar to COHb, heme molecules in MetHb are not only incapable of transporting oxygen molecules, but they also have an allosteric effect, increasing the affinity of the remaining heme groups for oxygen [20].
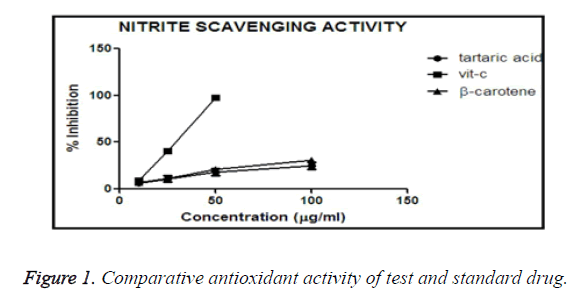
Inhibition of nitrite induced lysis of hRBC
hRBC produces NO (nitric oxide), a free radical, is produced due to presence of sodium nitrite. NO converts haemoglobin to methemoglobin which appears as a chocolate brown coloured blood. The more the concentration of the treatment drug, less in the formation of methemoglobin, results in lower absorbance. If the absorbance is higher, it confirms the drug has lower anti-oxidant activity.
All the test tubes were marked, and in different (0, 10, 25, 50, 100 μg/ml) concentrations of treatment drugs were mixed.
After incubation the absorbance was taken, and % inhibition was calculated accordingly. The values obtained are plotted in Graph Pad Prism-5 software, and the % inhibition vs. concentration of different molecules, i.e. tartaric acid, β- carotene, vitamin C drawn, that suggests the comparison of anti-oxidant activity.
Comparative antioxidant activity of β-carotene and tartaric acid and standard vitamin-C
From the graph it was obtained that, vitamin C shows maximum % inhibition in comparison to β-carotene and tartaric acid (Figure 1). Vitamin C shown the maximum potency to reduce the oxidative stress, and among our test compounds tartaric acid shown better scavenging activity than of β-carotene. That graph suggests that tartaric acid has more ability to reduce oxidative stress than β-carotene, so tartaric acid is having more anti-oxidant activity than β-carotene.
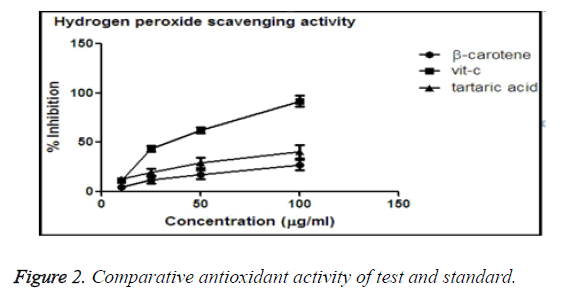
Hydrogen peroxide scavenging activity
Hydrogen peroxide is another free radical which exhibit oxidative stress on cell membrane [21]. In case of RBC the cell rigidity decreases due to influence of hydrogen peroxide, causing cell death. Our study molecules (Tartaric acid, β- carotene and vitamin C) which thought to have anti-oxida nt activity would protect the cell from lysis. All the test tubes were marked, and in different (0, 10, 25, 50, 100 μg/ml) concentrations of treatment drugs were mixed. After incubation the absorbance were taken, and % inhibition was calculated.
The values obtained were plotted in Graph Pad Prism-5 software to obtain the graphs of % inhibition vs. concentration of different molecules, i.e. tartaric acid, vitamin C which suggests the comparison of anti-oxidant activity.
Comparative antioxidant activity of β-carotene and tartaric acid and standard vitamin-C
From the graph it was obtained that, vitamin C shown maximum % inhibition in comparison to β-carotene and tartaric acid. Vitamin C shown the maximum potency to reduce the oxidative stress, and among test molecules tartaric acid shown better anti-oxidant activity than of β-carotene.
The IC50 values were calculated, from the obtained data. IC50 values describes about the minimum concentration needed to obtain the biological response.
From the Table 1 it is observed that the IC50 value of vitamin- C is lowest, so in minimum concentration vitamin-C can reach 50% inhibitory level. And among tartaric acid and β-carotene, tartaric acid requires lesser concentration than β-carotene to reach 50% of inhibitory level. So, antioxidant activity of tartaric acid is more than β-carotene (Figure 2).
| Name of the compound | IC50 value |
|---|---|
| Vitamin-C | 52.0982 ± 23.09902 |
| β-carotene | 194.65 ± 3.45 |
| Tartaric acid | 127.36 ± 2.45 |
Table 1: IC50 values.
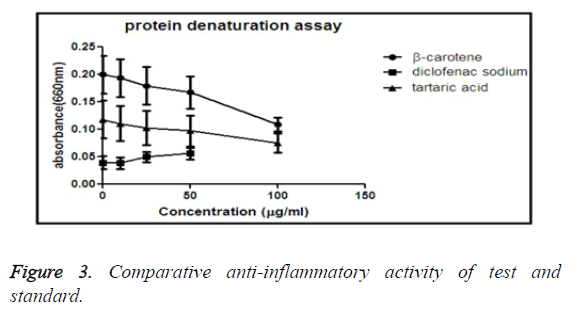
Protein denaturation assay
Denaturation of protein refers that the protein loses its secondary, quaternary or tertiary structure. The antioxidant’s role is to reduce the denaturation of proteins. In this experiment we are applying heat on BSA, so that the basic structure is ruptured, but the effect of anti-inflammatory activity of drug resists the denaturation.
Comparative anti-inflammatory activity by protein denaturation assay method of β-carotene and tartaric acid and standard diclofenac sodium
From the graph it is suggested that the standard drug diclofenac sodium shows the most anti-inflammatory activity than our experimental test drug. It is also clear that among the test drugs, tartaric acid has more ability to inhibit protein denaturation than β-carotene (Figure 3).
Discussion
Previously, the in vitro anti-oxidant activity was already been established [22] for β-carotene, here in this study the effect on human RBC was shown. The obtained results ensure the effectiveness of β-carotene to protect blood cell from cellular oxidation. Similarly the anti-oxidant activity of tartaric acid isolated from grape fruits was shown [9], but he comparative anti-oxidant and anti-inflammatory study was not established earlier. In our study protocol, it has been observed that both the molecules have good antioxidant activity to control cellular oxidation. They can be used as anti-inflammatory agent. The present study regarding, free radical scavenging assay of β- carotene and tartaric acid on hRBC by using vitamin-C as standard, the comparative reductive capacity on oxidative stress has been evaluated.
The result obtained after the study reflects tartaric acid is more effective antioxidant and anti-inflammatory agent than β- carotene on hRBC and as shown in establishing antiinflammatory activity by using standard drug. And so far it can be used as anti-rheumatoid arthritic agent as already discussed that rheumatoid arthritis is a degenerative autoimmune inflammatory disease.
Conclusion
After the study it can be concluded that the results found quite satisfactory for treatment of rheumatoid arthritis and inflammation and both the molecule has potent anti-oxidant potential at the same time. As the conventional medicines, available in market is costly and have several side effects, it is believed that treatment with phytomolecules would be a good alternative choice. The comparison study would be beneficial for selection of specific molecule. In future, if more phytomolecules are included in the study and compared, that will be more beneficial and the statistical value will be more acceptable.
References
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet 2009; 373: 659-672.
- Vishal V, Ganesh NS, Mukesh G, Ranjan B. Review on some plants having anti-inflammatory activity. J Phyto 2014; 3: 214-221.
- Giles GI, Jacob C. Reactive sulfur species: an emerging concept in oxidative stress. Biol Chem 2002; 383: 375-388.
- Ames BN, Shigenaga MK, Gold LS. DNA lesions, inducible DNA repair, and cell division: three key factors in mutagenesis and carcinogenesis. Environ Health Perspect 1993; 101: 35-44.
- Liu RH, Hotchkiss JH. Potential genotoxicity of chronically elevated nitric oxide: a review. Mutat Res 1995; 339: 73-89.
- Geier DA, Kern JK, Garver CR. A prospective study of transsulfuration biomarkersin autistic disorders. Neurochem Res 2009; 34: 386-393.
- Geier DA, Kern JK, Garver CR. Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci 2009; 280: 101-108.
- Windrow VR, Winyard PG, Morris CJ. Free radicals in inflammation: second messengers andmediators of tissue destruction. Free radicals in medicine. New York: Churchill. Living Stone 1993; 506-522.
- Boas ACV. Antioxidant activity, anthocyanins and organic acids content of grape juices produced in Southwest of Minas Gerais, Brazil. Ciênc Agrotec Lavras 2014; 5: 480-486.
- Helmy M, Shohayeb M, Helmy MH. Antioxidants as adjuvant therapy in rheumatoid disease. A preliminary study. Arzneimittelforschung 2001; 51: 293-298.
- Kamanli A, Naziroglu M, Aydile K. Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem Funct 2004; 22: 53-57.
- Mohanty JG. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol 2014; 5: 84.
- Kasthuri B. In vitro studies of antioxidant and antiinflammation activity of Argemone maxicana L. Flower Extract IJMCA 2014; 4: 79-82.
- Sainani GS, Manika JS, Sainani RG. Oxidative stress, a key factor in pathogenesis of chronic diseases. Med Update 1997; 1: 1.
- Mizushima Y, Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol 1968; 20: 169-173.
- Sakat S, Juvekar AR, Gambhire MN. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharm Sci 2010; 2: 146-156.
- Guyton AC, Hall JE. Textbook of medical physiology (11th Edn.). Philadelphia: Elsevier Saunders 2006.
- Linberg R, Conover CD, Shum KL, Shorr RG. Hemoglobin based oxygen carriers: how much methemoglobin is too much? Artif Cells Blood Substit Immobil Biotechnol 1998; 26: 133-148.
- Alain JMR. Structure-function relations of human hemoglobins. Proc (Bayl Univ Med Cent) 2006; 19: 239-245.
- Ruckman Jared S. A comparative study of total hemoglobin measurement technology: noninvasive pulse co-oximetry and conventional methods. Masters Teses Paper 2011; 75.
- Subhashinee SKW. Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in caco-2 human colon cells. J Agric Food Chem 2005; 53: 8768-8774.
- Mueller L, Boehm V. Antioxidant activity of β-carotene compounds in different in vitro assays. Molecules 2011; 16: 1055-1069.