ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2021) Volume 32, Issue 6
Combination therapy with metformin and the GLP-1 analogue liraglutide as a synergistic treatment for diabetic retinopathy in a type-2 diabetic rat model
Tourki A. S. Baokbah*
Department of Medical Emergency Services, Al-Qunfudah Health Sciences College, University of Umm Al-Qura, Makkah 21955, Saudi Arabia
- Corresponding Author:
- Tourki A. S. Baokbah
Department of Medical Emergency Services
Al-Qunfudah Health Sciences College
University of Umm Al-Qura
Makkah 21955
Saudi Arabia
E-mail: tabaokbah@uqu.edu.sa
Accepted date: September 28, 2021
This study investigated the effect of combination therapy with metformin and Glucagon-Like Peptide-1 (GLP-1) analogue, liraglutide, as a synergistic treatment for Diabetic Retinopathy (DR) in Type-2 Diabetes (T2DM). Normal rats and rats with established T2DM were divided equally into five groups. Group I: Normal non-diabetic rats, Group II: T2DM rats, Group III: T2DM rats administered metformin (30 mg/ kg) once daily for 12 weeks, Group IV: T2DM rats administered liraglutide (75 μg/kg) once daily for 12 weeks, and Group V: T2DM rats administered liraglutide (75 μg/kg) and metformin (30 mg/kg) once daily for 12 weeks. In each group, retinal morphology was observed using Haematoxylin-Eosin (H&E) staining. Lipid peroxidation (malondialdehyde) and endogenous antioxidant enzyme (catalase) levels were measured using a colorimetric method. Expression of autophagy (LC3&P62) and apoptosis (caspase-3) markers and Transforming Growth Factor-β (TGF-β) was detected using immunohistochemistry. H&E staining revealed that the T2DM group had evident retinal damage, which could be effectively improved using combination therapy with metformin and liraglutide compared with the two monotherapy treatments. Combined therapy improved the antioxidant ability of injured retinal tissue in the T2DM group by significantly (P?0.01) down regulating and restoring the levels of malondialdehyde and catalase, respectively. The low expression of TGF-β, LC3, and P62; and the high expression of caspase-3 in the retinal tissues of the T2DM group was significantly (P?0.01) reversed through combined treatment. Therefore, metformin combined with liraglutide synergistically attenuated DR in a T2DM rat model by inhibiting oxidative stress, apoptosis, and TGF-β expression while activating autophagy
Keywords
Diabetic retinopathy, Type-2 diabetes, Metformin, Liraglutide, Autophagy, Apoptosis, Transforming growth factor-β, Immunostaining
Introduction
Diabetes Mellitus (DM) is one of the most rapidly increasing chronic metabolic diseases in the word [1]. Globally, the number of people living with DM is projected to rise from 285 million in 2010 to 439 million by 2030 [2]. DM is characterised by abnormal and persistent hyperglycaemia [3] and is broadly classified into Type-1 (T1DM) and Type-2 (T2DM) DM, which account for approximately 5%-10% and 90%-95% of all DM cases, respectively [4]. T2DM is an age-and lifestyle-related disorder, defined by insulin resistance and pancreatic β-cell dysfunction, which is exacerbated by physical inactivity and obesity [5].
Diabetic Retinopathy (DR) is one of the most common complications of DM, characterised by retinal damage in individuals with long-term DM [6]. It is now the leading cause of visual impairment and blindness among middle-aged and elderly people worldwide [7]. DR is defined by changes in capillary microaneurysms, capillary obstruction, retinal degeneration caused by ischaemia, and an abnormal increase in the formation of new blood vessels (neovascularisation) within the retina. The newly formed blood vessels are fragile, allowing fluid and blood to leak into the retina, resulting in macular oedema and visual impairment [8]. The onset of DR is primarily determined by the severity and duration of hyperglycaemia [7].
Autophagy is an evolutionarily conserved homeostatic process in which intracellular proteins and damaged organelles are degraded and recycled to maintain normal cellular function [9]. Several studies have implicated autophagy in the maintenance of β-cell function and survival under normal conditions and as an adaptive mechanism under stress conditions by preventing β-cell dysfunction or death [10,11]. Autophagy dysfunction, due to chronic hyperglycaemia, has been linked to the progression of T2DM [12,13] and shown to play a significant role in T2DM-related complications, such as neuropathy [14], cardiomyopathy [15], and retinopathy [16].
Retinal cells undergo apoptosis in response to various noxious stimuli, including diabetic stress [17]; however, autophagy has also been implicated in the fate of stressed retinal cells [18]. In DR, autophagy appears to play a cytoprotective role, allowing retinal cells to cope with stress conditions by preventing apoptosis [16]. However, prolonged diabetic stress may cause dysfunctional autophagy and increased susceptibility to apoptosis [16,19].
The cytokine Transforming Growth Factor-β (TGF-β) is abundantly expressed in the eyes and can be abnormally overexpressed in response to high blood glucose levels [20,21]. Levels of TGF-β are abnormally high in the serum of patients with diabetes; levels increase as DR progresses, implying that TGF-β is involved in the aetiology of DR [20]. Consistently, TGF-β-mediated signalling regulates retinal tissue fibrosis which plays a critical role in DR pathogenesis [20].
Glucagon-Like Peptide-1 (GLP-1) is an incretin hormone produced by enteroendocrine L-cells in the ileum, small intestine, and colon following food consumption [22]. GLP-1 acts as a hypoglycaemic agent by increasing insulin secretion from β-cells and inhibiting glucagon release [23]. GLP-1 exerts other biological effects, including reducing fasting and postprandial lipid levels in healthy and T2DM patients as well as causing weight loss and blood pressure reduction by binding to its receptor, GLP-1R [24]. Patients with T2DM have a significantly reduced incretin effect, and treatment with a GLP-1 analogue, such as liraglutide, improved glycaemic control by increasing insulin secretion while decreasing glucagon release and delaying gastric emptying [24]. GLP-1 analogues have been reported to stimulate autophagy in β-cells and improve their function, protecting them from glucolipotoxicity [25,26]. GLP-1 analogues are primarily used with metformin in patients with T2DM who do not respond adequately to metformin [27]. Metformin, the first-line oral therapy for T2DM, is typically prescribed to individuals who do not achieve adequate glycaemic control through lifestyle changes alone. It primarily works by increasing insulin sensitivity and decreasing hepatic glucose production [28]. In addition, the protective effect of metformin against β-cell death has been linked to an increase in autophagy [29].
The aim of this study is to investigate the effect of combination therapy with metformin and liraglutide as a synergistic treatment for DR in a T2DM rat model.
Materials and Methods
Experimental animals
Forty adult male albino rats, aged 10 weeks (weighing 220–250 g), were obtained from the animal house of the Faculty of Agriculture, Mansoura University. The rats were housed in separate cages under standard experimental conditions (12 h day/night cycle and 22ºC) with free access to water and food. All experimental procedures for this study were approved by the ethics committee of Mansoura Medical College.
Establishment of diabetic retinopathy in the T2DM rat model
T2DM induction in rats was performed as described [30]. Briefly, rats were fed a high-fat diet with a total calorie content of 40 kJ/kg (20% fat, 45% carbohydrate, and 22% protein) for 12 weeks. Four weeks after the start of the experiment, a single dose of Streptozotocin (STZ) (35 mg/kg) (Sigma-Aldrich, St Louis, MO, USA) was administered intraperitoneally (i.p.). T2DM was confirmed by measuring blood sugar levels in blood from the tail vein after 48 h of STZ treatment. Rats with blood glucose levels exceeding 200 mg/dl were included in this study as a T2DM model. DR was confirmed by alterations in retinal morphology as pyknotic nuclei and diffuse vacuolation.
Study design
Group I: Normal control group: Normal non-diabetic rats were given 0.5 ml saline via gastric gavage and 0.5 ml saline via Subcutaneous (S.C.) injection once daily for 12 weeks.
Group II: Established T2DM group: T2DM rats were administered saline (0.5 ml) via gastric gavage and saline (0.5 ml) via S.C. injection once daily for 12 weeks.
Group III: T2DM+metformin group: T2DM rats were administered metformin (30 mg/kg) (Sigma, USA) [31] prepared in 0.5 ml saline by oral gavage and 0.5 ml saline via S.C. injection once daily for 12 weeks.
Group IV: T2DM+liraglutide group: T2DM rats were administered liraglutide (Novo Nordisk A.S, Denmark) 75 μg/kg [32] prepared in 0.5 ml saline via S.C. injection and 0.5 ml saline via gastric gavage once daily for 12 weeks.
Group V: T2DM+liraglutide+metformin group: T2DM rats were administered liraglutide (75 μg/kg) via S.C. injection and metformin (30 mg/kg) by oral gavage once daily for 12 weeks.
The animals were anaesthetised with an intraperitoneal injection of pentobarbital (Nervicanis, Egypt) (0.1 mg/g) and euthanised 24 h after the last treatment. Retinal tissues were separated after the eyeballs were removed.
Measurement of lipid peroxidation and catalase levels in retinal tissues
Retinal tissues from all groups were homogenised in 1 ml cold buffer (50 mM potassium phosphate [pH 7.5] and 1 mM EDTA) using a mortar and pestle. The retina homogenate was then centrifuged at 4,000 × g for 15 min at 4ºC, and the supernatant was stored at -20ºC for analysis of Malondialdehyde (MDA) concentration and catalase (CAT) activity using a colorimetric method in accordance with the manufacturer's recommendations (Bio Diagnostics, Dokki, Giza, Egypt).
Histological and immunohistochemical staining
Retinal tissue samples from all groups were fixed using 10% paraformaldehyde, dehydrated through an ascending gradient of ethanol, cleared in xylene, and embedded in paraffin blocks [33]. Tissue samples were then cut into 3.0–4.0 μm thick sections using a manual rotary microtome knife (Leica RM2235, Leica Biosystems, UK) mounted on superfrost/Plus glass slides (Fisher Scientific, UK). For histological observation, the tissue sections were stained with Haematoxylin and Eosin (H&E) [33]. Briefly, tissue sections were deparaffinised in xylene and rehydrated through a descending gradient of ethanol at room temperature (22–24ºC). Subsequently, the sections were washed in distilled water, stained at room temperature (22–24ºC) with haematoxylin solution for 10 min, washed in running tap water, and soaked in 1% acid-alcohol for 30 s at room temperature (22–24ºC) for differentiation. Then, the tissue sections were washed in running tap water for 5 min, stained with eosin solution for 30 s at room temperature (22–24ºC), dehydrated through an ascending gradient of ethanol, cleared in xylene, and mounted in Dibutylphthalate Polystyrene Xylene (DPX) mounting media.
The expression of LC3, P62, caspase-3, and TGF-β was detected using a biotinylated goat anti polyvalent HRP/DAB (ABC) detection IHC kit (ab64264, Abcam, Cambridge, United Kingdom). Briefly, tissue sections were deparaffinised in xylene, rehydrated through a descending ethanol gradient, and subjected to antigen retrieval in 10 mM citrate buffer (pH 6) at 100ºC for 1 h [34]. The sections were then cooled at room temperature (22–24ºC), washed three times in Phosphate-Buffered Saline (PBS), and covered with 3% H2O2 (ab64264, Abcam) for 10 min at room temperature (22–24ºC) to inactivate endogenous peroxidase activity. Tissue sections were then washed three times in PBS and incubated with blocking protein (ab64264, Abcam) for 10 min at room temperature (22– 24ºC) to block nonspecific binding. Subsequently, the sections were incubated with a specific primary antibody against anti-caspase 3 (rabbit polyclonal antibody, NeoMarkers, Fremont. CA, USA) at dilution (1:100); anti LC3B antibody (Abcam Rabbit monoclonal # ab192890) at dilution (1:100); anti P62 (purchased from Abcam Rabbit monoclonal # ab109012) at dilution (1:100), and mouse anti TGF-β monoclonal antibody, dilution 3:1000 (DAKO) overnight at 4ºC. The next day, sections were rinsed, incubated with biotinylated goat antipolyvalent (ab64264, Abcam) for 1 h at room temperature (22–24ºC), rinsed, and incubated with the streptavidin-peroxidase solution for 10 min. Coloured signal was developed using diaminobenzidine (ab64264, Abcam). Finally, the sections were counterstained with haematoxylin, hydrated, and mounted in DPX. Histological changes and immunohistochemical staining were observed under a light microscope (Leica DM500 with Leica ICC50 HD camera and Leica LAS EZ imaging software version 3.1.1, Leica Biosystems, UK) in at the Anatomy Department, Faculty of Medicine, Mansoura University. Immunostaining in the retina tissue (region of interest) was quantified as a percentage of positive staining (calculated by averaging the values from 10 fields at 10× magnification) for each retinal area using the Image J (NIH) software (version 1.33) [35].
Statistical analysis
Statistical significance of different groups was analysed using analysis of variance (ANOVA) and Tukey’s post hoc multiple comparison using SPSS software version 17. All data are represented as mean ± SEM (n=4). Differences were considered statistically significant at P<0.05. Statistical significance was set at P<0.05.
Results
The effect of separate and combined treatment with metformin and liraglutide on retinal morphology
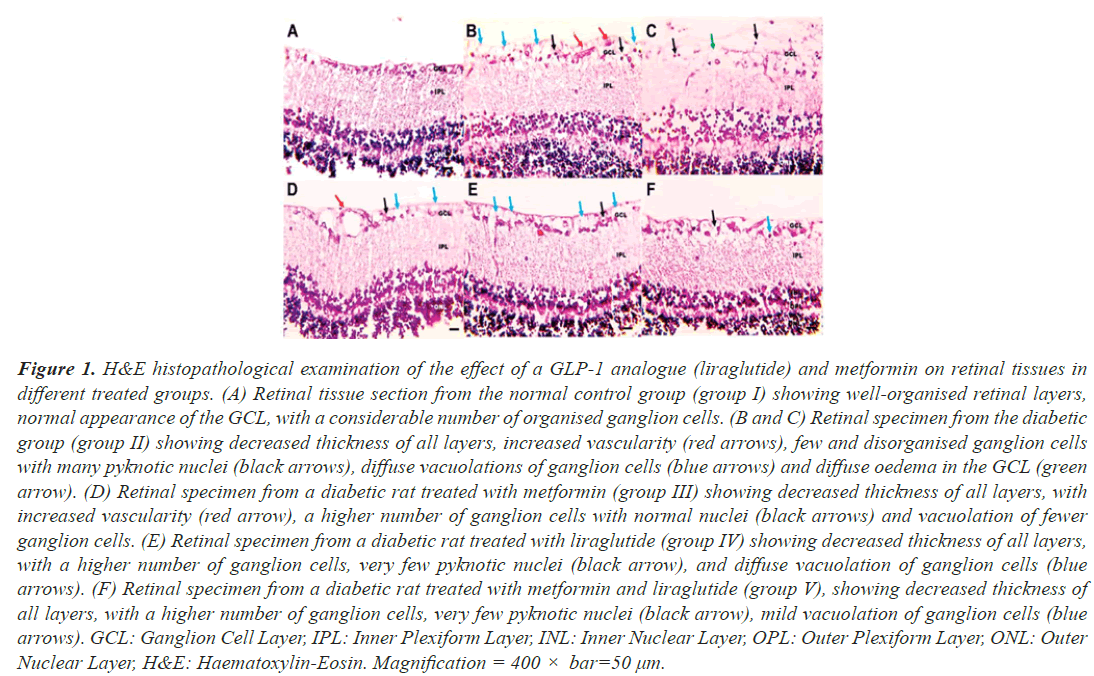
Histopathological examination of retinal tissue sections from control rats (group I) was performed using H&E staining, which showed normal histological features, with well-organised retinal layers and a normal appearance of the Ganglion Cell Layer (GCL) with a considerable number of organised ganglion cells (Figure 1A). Retinal tissues obtained from T2DM rats (group II) showed typical characteristics of DR as a decrease in the thickness of all layers of the retina, with increased vascularity, and the GCL showed many pyknotic nuclei, diffuse vacuolation, and diffuse oedema (Figures 1B and 1C). In contrast, retinal tissues obtained from diabetic rats who received a combination of metformin and liraglutide (group V), showed very few pyknotic nuclei and vacuolations, with improved thickness of all layers (Figure 1F), whereas diabetic rats who received metformin (group III) and liraglutide (group IV) as monotherapy moderate, pyknotic nuclei, and vacuolations with increased vascularity (Figure 1D and 1E). This indicates that combined treatment with metformin and liraglutide improves the morphology of the retinal tissue in diabetic rats.
Figure 1: H&E histopathological examination of the effect of a GLP-1 analogue (liraglutide) and metformin on retinal tissues in different treated groups. (A) Retinal tissue section from the normal control group (group I) showing well-organised retinal layers, normal appearance of the GCL, with a considerable number of organised ganglion cells. (B and C) Retinal specimen from the diabetic group (group II) showing decreased thickness of all layers, increased vascularity (red arrows), few and disorganised ganglion cells with many pyknotic nuclei (black arrows), diffuse vacuolations of ganglion cells (blue arrows) and diffuse oedema in the GCL (green arrow). (D) Retinal specimen from a diabetic rat treated with metformin (group III) showing decreased thickness of all layers, with increased vascularity (red arrow), a higher number of ganglion cells with normal nuclei (black arrows) and vacuolation of fewer ganglion cells. (E) Retinal specimen from a diabetic rat treated with liraglutide (group IV) showing decreased thickness of all layers, with a higher number of ganglion cells, very few pyknotic nuclei (black arrow), and diffuse vacuolation of ganglion cells (blue arrows). (F) Retinal specimen from a diabetic rat treated with metformin and liraglutide (group V), showing decreased thickness of all layers, with a higher number of ganglion cells, very few pyknotic nuclei (black arrow), mild vacuolation of ganglion cells (blue arrows). GCL: Ganglion Cell Layer, IPL: Inner Plexiform Layer, INL: Inner Nuclear Layer, OPL: Outer Plexiform Layer, ONL: Outer Nuclear Layer, H&E: Haematoxylin-Eosin. Magnification = 400 × bar=50 µm.
The effect of separate and combined treatment with liraglutide and metformin on MDA and CAT levels in retinal tissues
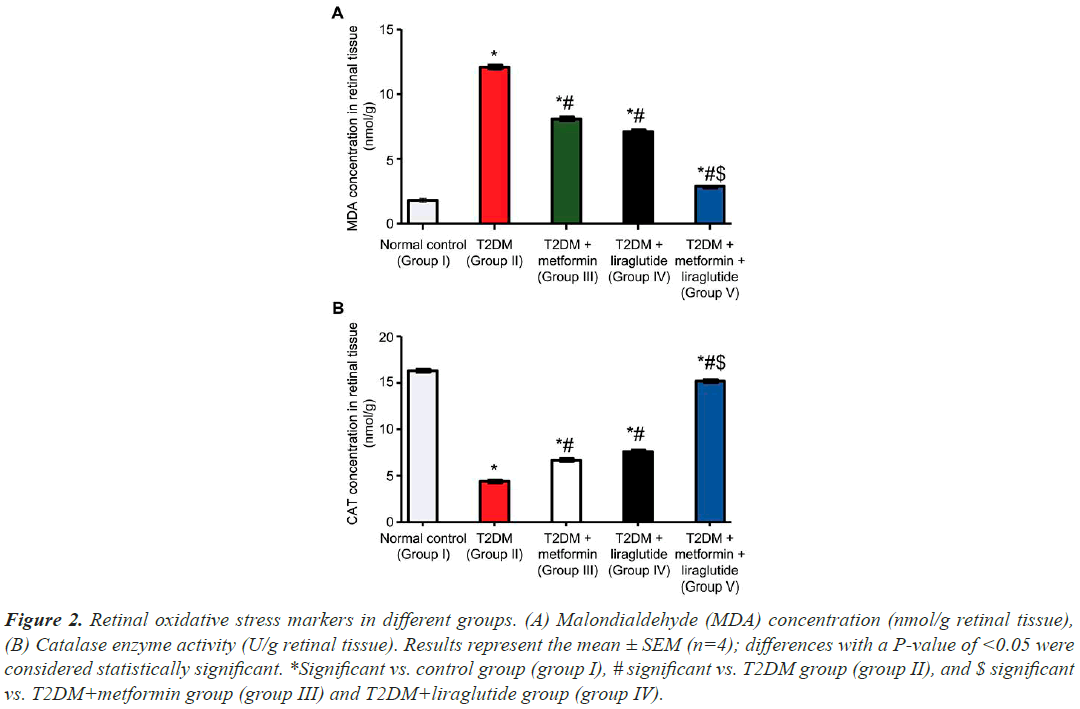
MDA, a by-product of polyunsaturated fatty acid peroxidation in cells, is frequently used as an indicator of oxidative stress. To determine the effect of separate and combined treatment with metformin and liraglutide on oxidative stress in retinal tissues in T2DM rats, MDA levels were measured in different groups (Figure 2A). MDA levels in the retinal tissues of the T2DM group (group II) were significantly higher (P˂0.001) than those in the normal control group (group I). In T2DM groups treated with metformin (group III) or liraglutide (group IV), there was less significant reduction (P˂0.05) in MDA levels compared with the T2DM group. However, this reduction in MDA levels was most pronounced in the T2DM group treated with a combination of liraglutide and metformin (P˂0.01). The concentration of CAT was also measured in different groups (Figure 2B). CAT levels were significantly lower in the T2DM group than in the normal control group (P˂0.001). However, in diabetic rats treated with metformin (group III) or liraglutide (group IV), there was a less significant increase in CAT levels (P˂0.05) compared with the T2DM group. This increase in CAT levels was most pronounced (P˂0.01) in the T2DM group treated with a combination of both metformin and liraglutide (group V).
Figure 2: Retinal oxidative stress markers in different groups. (A) Malondialdehyde (MDA) concentration (nmol/g retinal tissue), (B) Catalase enzyme activity (U/g retinal tissue). Results represent the mean ± SEM (n=4); differences with a P-value of <0.05 were considered statistically significant. *Significant vs. control group (group I), # significant vs. T2DM group (group II), and $ significant vs. T2DM+metformin group (group III) and T2DM+liraglutide group (group IV).
The effect of separate and combined treatment with metformin and liraglutide on autophagy markers LC3 and P62 in retinal tissues
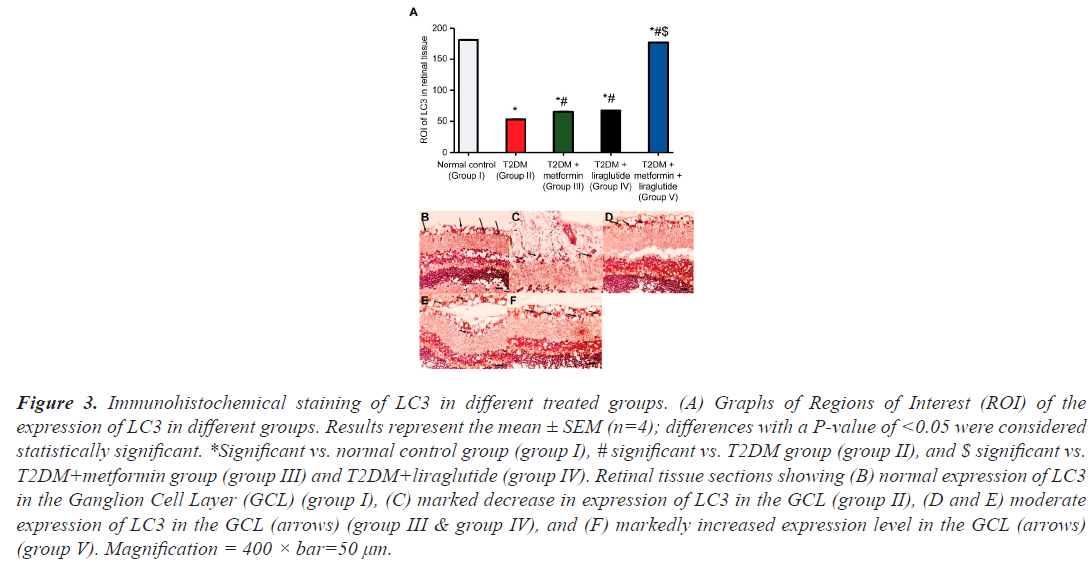
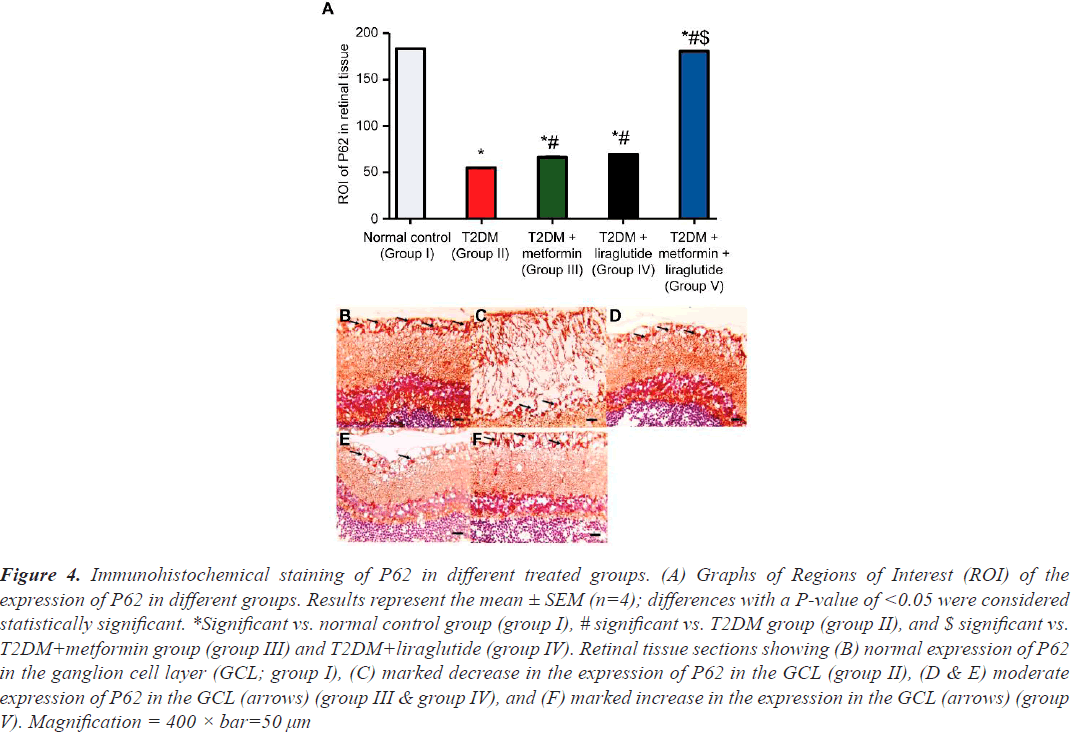
To determine the effect of metformin, liraglutide, and a combination of both on retinal autophagy, the expression of LC3 and P62 was assessed using immunohistochemistry (Figures 3 and 4). A high number of LC3 (Figure 3B) and P62 (Figure 4B) positive cells in the GCL were observed in the retinal sections of the normal control group (group I), whereas in the T2DM group (group II), a few LC3 (Figure 3C) and P62 (Figure 4C) positive cells, compared with that in group I, were detected. In the T2DM group treated with metformin (group III) or liraglutide (group IV), a moderate increase was observed in the number of LC3 (Figure 3D and 4E), and P62 (Figure 3D and 4E) positive cells compared with that in group II. However, in the T2DM group treated with a combination of both metformin and liraglutide (group V), large numbers of LC3 (Figure 3F) and P62 positive cells (Figure 4F) were observed in the GCL compared with that in group II. Quantification of the expression of LC3 (Figure 3A) and P62 (Figure 4A) revealed a highly significant reduction (P˂0.001) of LC3 and P62 expression in group II compared with that in group I, and a moderate, but less significant (P˂0.05), increase in the expression of LC3 and P62 in groups III and IV compared with that in group II. However, in group V, a significant increase in the expression (P˂0.01) of LC3 and P62 was observed in the GCL compared with that in group II.
Figure 3: Immunohistochemical staining of LC3 in different treated groups. (A) Graphs of Regions of Interest (ROI) of the expression of LC3 in different groups. Results represent the mean ± SEM (n=4); differences with a P-value of <0.05 were considered statistically significant. *Significant vs. normal control group (group I), # significant vs. T2DM group (group II), and $ significant vs. T2DM+metformin group (group III) and T2DM+liraglutide (group IV). Retinal tissue sections showing (B) normal expression of LC3 in the Ganglion Cell Layer (GCL) (group I), (C) marked decrease in expression of LC3 in the GCL (group II), (D and E) moderate expression of LC3 in the GCL (arrows) (group III & group IV), and (F) markedly increased expression level in the GCL (arrows) (group V). Magnification = 400 × bar=50 µm.
Figure 4: Immunohistochemical staining of P62 in different treated groups. (A) Graphs of Regions of Interest (ROI) of the expression of P62 in different groups. Results represent the mean ± SEM (n=4); differences with a P-value of <0.05 were considered statistically significant. *Significant vs. normal control group (group I), # significant vs. T2DM group (group II), and $ significant vs. T2DM+metformin group (group III) and T2DM+liraglutide (group IV). Retinal tissue sections showing (B) normal expression of P62 in the ganglion cell layer (GCL; group I), (C) marked decrease in the expression of P62 in the GCL (group II), (D & E) moderate expression of P62 in the GCL (arrows) (group III & group IV), and (F) marked increase in the expression in the GCL (arrows) (group V). Magnification = 400 × bar=50 µm
The effect of separate and combined treatment with metformin and liraglutide on the apoptotic marker caspase-3 in retinal tissue
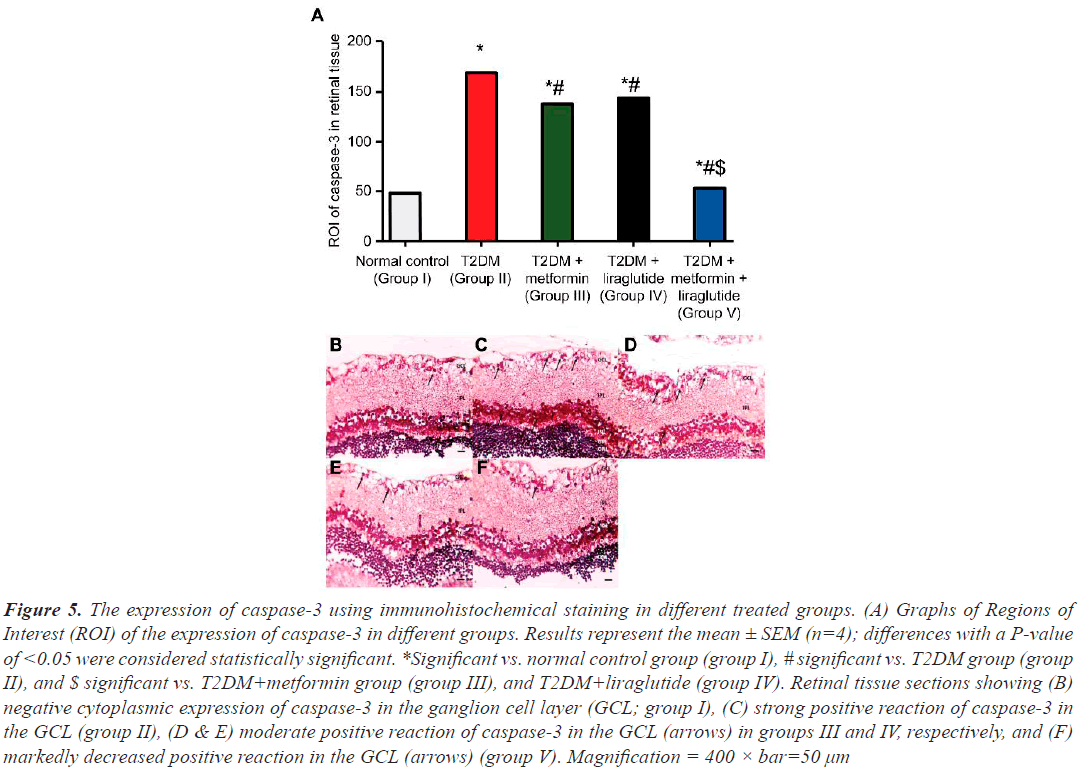
The incidence of apoptosis in different groups was investigated by determining the expression of caspase-3 in different groups (Figure 5). Immunohistochemically stained sections revealed few caspase-3 positive cells in the GCL in the retinal sections of the normal control group I (Figure 5B and 5A). In contrast, in the T2DM group (group II), a large and highly significant increase (P˂0.001) in caspase-3 positive cells compared with that in group I (Figure 5C and 5A) was observed. In the T2DM group treated with metformin (group III) or liraglutide (group IV), a moderate but less significant (P˂0.05) decrease in the number of caspase-3 positive cells was observed compared with that in group II (Figure 5D and 5E). However, in the T2DM group treated with a combination of both metformin and liraglutide (group V), a significant reduction in the expression (P˂0.01) of caspase-3 was observed in the GCL compared with that in group II (Figure 5F and 5A).
Figure 5: The expression of caspase-3 using immunohistochemical staining in different treated groups. (A) Graphs of Regions of Interest (ROI) of the expression of caspase-3 in different groups. Results represent the mean ± SEM (n=4); differences with a P-value of <0.05 were considered statistically significant. *Significant vs. normal control group (group I), # significant vs. T2DM group (group II), and $ significant vs. T2DM+metformin group (group III), and T2DM+liraglutide (group IV). Retinal tissue sections showing (B) negative cytoplasmic expression of caspase-3 in the ganglion cell layer (GCL; group I), (C) strong positive reaction of caspase-3 in the GCL (group II), (D & E) moderate positive reaction of caspase-3 in the GCL (arrows) in groups III and IV, respectively, and (F) markedly decreased positive reaction in the GCL (arrows) (group V). Magnification = 400 × bar=50 µm
The effect of separate and combined treatment with metformin and liraglutide on TGF-β in retinal tissue
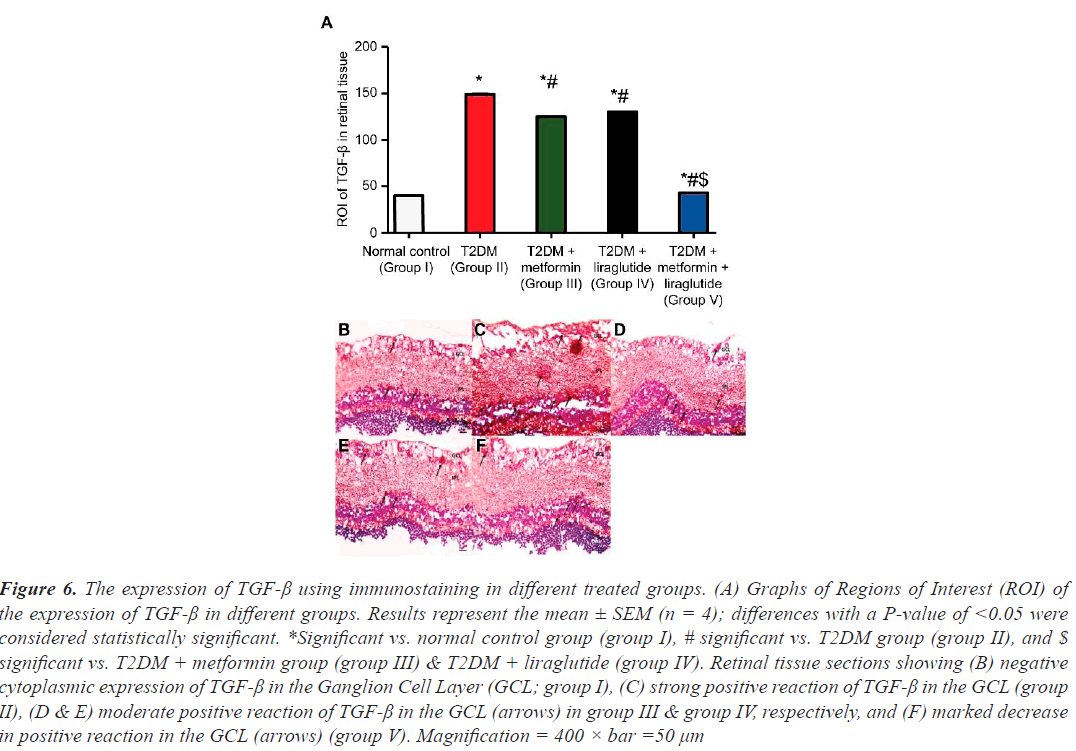
Examining the effect of separate and combined treatment with metformin and liraglutide on the expression of TGF-β revealed a few cells that stained positive for TGF-β in the GCL in retinal sections of the normal control group I (Figure 6A and 6B), whereas in the T2DM group (group II), a large and highly significant increase (P˂0.001) of TGF-β-positive cells was observed compared with that in group I (Figure 6C and 6A). In the T2DM group treated with metformin (group III) or liraglutide (group IV), a moderate but less significant (P˂0.05) decrease in the number of TGF-β-positive cells compared with that in group II was observed (Figure 6D and 6E). However, in the T2DM group treated with a combination of metformin and liraglutide (group V), a significant reduction in TGF-β expression (P˂0.01) was observed in the GCL compared with that in group II (Figure 6F and 6A).
Figure 6: The expression of TGF-ß using immunostaining in different treated groups. (A) Graphs of Regions of Interest (ROI) of the expression of TGF-ß in different groups. Results represent the mean ± SEM (n = 4); differences with a P-value of <0.05 were considered statistically significant. *Significant vs. normal control group (group I), # significant vs. T2DM group (group II), and $ significant vs. T2DM + metformin group (group III) & T2DM + liraglutide (group IV). Retinal tissue sections showing (B) negative cytoplasmic expression of TGF-ß in the Ganglion Cell Layer (GCL; group I), (C) strong positive reaction of TGF-ß in the GCL (group II), (D & E) moderate positive reaction of TGF-ß in the GCL (arrows) in group III & group IV, respectively, and (F) marked decrease in positive reaction in the GCL (arrows) (group V). Magnification = 400 × bar =50 µm
Discussion
T2DM is the most prevalent form of DM in adults, defined by hyperglycaemia caused by peripheral insulin resistance and islet β-cell dysfunction [4]. One complication of uncontrolled chronic DM is DR, a neurological and vascular disease of the retina that results in severe and sometimes irreversible vision loss [6]. Given that liraglutide in combination with metformin is used to treat T2DM [36] and each drug individually has a protective effect against DR [37–39], the primary goal of this study was to investigate the effect of combination therapy with metformin and liraglutide as a synergistic treatment for DR in a T2DM rate model.
First, a T2DM rat model was developed as described [30], and H&E-stained sections of retinal tissues were examined for pathological changes. A disordered arrangement of retinal ganglion cells with altered morphology, numerous pyknotic nuclei, diffuse vacuolation, and diffuse oedema were observed, indicating widespread apoptosis, as shown by the substantial increase in caspase-3 overexpression. These morphological changes, along with the high rate of apoptosis, are consistent with retinal tissue injury associated with DR [40,41].
To determine whether combined therapy protects against DR, pathological changes in the retinal tissues of rats in each treatment group were observed. Compared with T2DM control rats, T2DM rats treated with combination therapy had more orderly arranged retinal ganglion cells with improved morphology, a greater number of intact ganglion cells, no increase in vascularity, very few pyknotic nuclei, mild vacuolation of ganglion cells, and no oedema. Furthermore, this improvement in morphology was accompanied by a decrease in retinal cell apoptosis, as measured by low caspase-3 expression. Thus, the combined effect of metformin and liraglutide on T2DMinduced retinal tissue injury appears to inhibit retinal ganglion cell apoptosis and provides significantly greater protection than monotherapy with either.
Besides hyperglycaemia, oxidative stress is also a critical initiating factor in the development of DR [42]. DR rat models display damaged retinal mitochondria, resulting in abnormal production of Reactive Oxygen Species (ROS) and oxidative stress [43]. Several studies have also demonstrated that elevated levels of ROS are associated with DR, and there is strong evidence that the activity of antioxidant enzymes, such as CAT, decreases in the retina of patients with diabetes [44,45]. Furthermore, oxidative stress is detrimental to retinal ganglion cells as it accelerating apoptosis [46–48]. In this study, the expression levels of MDA and CAT, which are indicators of the extent of damage and protection against oxidative stress, respectively, were measured to determine whether a combination treatment could effectively improve the antioxidant capacity of the retinal tissue of T2DM rats. Consistent with previous reports [44,45], a notable increase in MDA levels and a decrease in endogenous antioxidant enzyme CAT levels were observed in the retinal tissues of T2DM rats. When compared with the two monotherapy treatment groups, combined treatment effectively reduced and restored the levels of MDA and CAT, respectively, demonstrating that combination therapy has the potential to ameliorate oxidative stress-induced injury in the retina of T2DM rats. These findings might explain the significant reduction in the incidence of retinal cell apoptosis.
The TGF-β-mediated signalling pathway is critical in the development of DR. It is directly involved in tissue fibrosis by promoting fibroblast proliferation [20,49]. Studies have shown that TGF-β can be linked to the development of retinal fibrotic events [50,51]. Our findings that TGF-β is significantly more abundant in the retinal tissues of T2DM rats than in those of normal rats, support those of another study [50], implying that TGF-β signalling may be activated in the retinal tissues of T2DM rats to induce fibrosis. Fibrosis develops late in the pathogenesis of DR, altering the retinal architecture and disrupting normal cellular relationships, resulting in vision impairment [50,51]. Once established, it is irreversible, and there is no preventative or curative treatment. In this study, the combination of metformin and liraglutide significantly reduced TGF-β overexpression compared with either therapy alone, suggesting a potential therapy for preventing retinal fibrosis.
Autophagy is a cellular defence mechanism that involves the degradation and recycling of organelles and proteins to maintain cellular homeostasis [9]. It is becoming increasingly recognised as an important factor in the pathogenesis of diabetes and its complications [11,12,16]. Impaired autophagy in β-cells because of multiple stressors, such as chronic hyperglycaemia, hyperlipidaemia, and oxidative stress, has been linked to the progression of T2DM [12,13]. Studies have demonstrated that autophagy is a two-edged sword, with mild conditions promoting cell survival and chronic and acute severe stress resulting in dysfunctional autophagy and an increase in retinal cells apoptosis [16–19]. Our findings reveal low autophagy in retinal tissues in T2DM rats, as measured by the low expression of the early and late autophagy markers LC3 and P62, respectively. Furthermore, retinal tissues from T2DM rats showed a marked increase in apoptosis, as indicated by a significant increase in caspase-3 expression. These findings suggest that autophagic processes may be overwhelmed or impaired because of chronic hyperglycaemia, resulting in excessive cell loss through caspase-3-mediated apoptosis. Moreover, there is some evidence that metformin, the first-line drug for T2DM, and novel anti-diabetic agents, such as GLP-1 analogues, can modulate autophagy [26,52,53]. Combination treatment with metformin and liraglutide significantly increased the expression of LC3 and P62 in the retinal tissues of T2DM rats, accompanied by a reduction in apoptosis when compared with the two monotherapy treatment groups, confirming the protective role of autophagy and the ability of both drugs to regulate autophagy.
In conclusion, the combination of metformin and liraglutide was significantly more effective than monotherapy in alleviating DR in a T2DM rat model by inhibiting oxidative stress, apoptosis, and TGF-β expression as well as activating autophagy, and holds promise for preventing diabetic complications associated with DR.
Acknowledgments
The author would like to thank colleagues at the Anatomy Department of Mansoura Medical College, University of Mansoura, Egypt, for their technical assistance. I am also grateful to the University for providing their database and resources for use in the research.
Disclosure Statement
The author declares no conflict of interest.
Funding
This study did not receive funding from any source.
References
- Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology 1998; 105: 998–1003.
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14.
- Blair M. Diabetes mellitus review. Urol Nurs 2016; 36: 27–36.
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2017; 40: S11–S24.
- Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 2011; 378: 169–181.
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010; 376: 124–136.
- Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: A review. Clin Exp Ophthalmol 2016; 44: 260–277.
- Eshaq RS, Aldalati AMZ, Harris NR, Alexander SA. Diabetic retinopathy: Breaking the barrier. Pathophysiology 2017; 24: 229–241.
- Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol 2015; 16: 461–472.
- Riahi Y, Wikstrom JD, Bachar-Wikstrom E. Autophagy is a major regulator of beta cell insulin homeostasis. Diabetologia 2016; 59: 1480–1491.
- Hayes HL, Peterson BS, Haldeman JM. Delayed apoptosis allows islet β-cells to implement an autophagic mechanism to promote cell survival. PloS One 2017; 12: e0172567.
- Yao D, GangYi Y, QiNan W. Autophagic dysfunction of B cell dysfunction in type 2 diabetes, a double-edged sword. Genes Dis 2020; 8: 438–447.
- Rocha M, Apostolova N, Diaz-Rua R. Mitochondria and T2D: Role of Autophagy, ER Stress, and Inflammasome. Trends Endocrinol Metab 2020; 31: 725–741.
- Zeng Q, Siu W, Li L. Autophagy in Alzheimer's disease and promising modulatory effects of herbal medicine. Exp Gerontol 2019; 119: 100–110.
- Ren J, Sowers JR, Zhang Y. Metabolic stress, autophagy, and cardiovascular aging: from pathophysiology to therapeutics. Trends Endocrinol Metab 2018; 29: 699–711.
- Lopes de Faria JM, Duarte DA, Montemurro C. Defective autophagy in diabetic retinopathy. Invest Ophthalmol Vis Sci 2016; 57: 4356–4366.
- Chinskey ND, Besirli CG, Zacks DN. Retinal cell death and current strategies in retinal neuroprotection. Curr Opin Ophthalmol 2014; 25: 228–233.
- Russo R, Berliocchi L, Adornetto A. In search of new targets for retinal neuroprotection: is there a role for autophagy? Curr Opin Pharmacol 2013; 13: 72–77.
- Yao J, Tao ZF, Li CP. Regulation of autophagy by high glucose in human retinal pigment epithelium. Cell Physiol Biochem 2014; 33: 107–116.
- Dagher Z, Gerhardinger C, Vaz J. The Increased Transforming Growth Factor-β Signaling Induced by Diabetes Protects Retinal Vessels. Am J Pathol 2017; 187: 627–638.
- Yue Y, Meng K, Pu Y, Zhang X. Transforming Growth Factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract 2017; 133: 124–130.
- Holst JJ. The physiology of glucagon-like peptide-1. Physiol Rev 2007; 87: 1409–1439.
- Meloni AR, DeYoung MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic b-cells: mechanism and glucose dependence. Diabetes Obes Metab 2013; 15: 15–27.
- Muscogiuri G, DeFronzo RA, Gastaldelli A, Holst JJ. Glucagon-like peptide-1 and the central/peripheral nervous system: crosstalk in diabetes. Trends Endocrinol Metab 2017; 28: 88–103.
- De Graaf C, Donnelly D, Wootten D, et al. Glucagon-like peptide-1 and its Class B G protein–coupled receptors: a long march to therapeutic successes. Pharmacol Rev 2016; 68: 954–1013.
- Ashrafizadeh M, Yaribeygi H, Atkin SL, Sahebkar A. Effects of newly introduced antidiabetic drugs on autophagy. Diabetes Metab Syndr 2019; 13: 2445–2449.
- Ahrén B. GLP-1 receptor agonists in the treatment of Type 2 diabetes. Diabetes Manage 2013; 3: 401–413.
- Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in Type 2 diabetes: a systematic review. Diabetologia 2006; 49: 434–441.
- Li Q, Jia S, Xu L, Li B, Chen N. Metformin‐induced autophagy and irisin improves INS‐1 cell function and survival in high‐glucose environment via AMPK/SIRT1/PGC‐1α signal pathway. Food Sci Nutr 2019; 7: 1695–1703.
- Hussein AEM, Omar NM, Sakr H, Elsamanoudy AZ, Shaheen D. Modulation of Metabolic & Cardiac Dysfunctions by Insulin Sensitizers and Angiotensin Receptor Blocker in Rat Model of Type 2 Diabetes Mellitus. Can J Physiol Pharmacol 2011; 89: 216–226.
- Makar NN, Elhawary AM, Emam HT, Abo Ria NH, Shaaban E. Possible Beneficial Effect of Metformin Alone or in Combination with Methotrexate in Rheumatoid Arthritis Induced Rat Model. Benha Med J 2019; 37: 143–154.
- Yang Y, Fang H, Xu G. Liraglutide improves cognitive impairment via the AMPK and PI3K/Akt signaling pathways in type 2 diabetic rats. Mol Med Rep 2018; 18: 2449–2457.
- Drury R, Wallington E. 1976. Carleton’s Histological Technique. Oxford University Press, London.
- Buchlowalow BI, Bocker W. Immunohistochemistry. Basics and Methods. Springer Verlag, Berlin Heidelberg, 2010; 48.
- Hussein AM, Eid EA, Taha M, et al. Comparative Study of the Effects of GLP1 Analog and SGLT2 Inhibitor against Diabetic Cardiomyopathy in Type 2 Diabetic Rats: Possible Underlying Mechanisms. Biomed 2020; 8: 43.
- Udiawar MV, Bain SC. Liraglutide in Combination with Metformin or Sulfonylurea for the Treatment of Type 2 Diabetes. Clin Med Insights Ther 2012; 4: 251–261.
- Hernández C, Bogdanov P, Corraliza L. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016; 65: 172–187.
- Fan YP, Wu CT, Lin JL. Metformin Treatment Is Associated with a Decreased Risk of Nonproliferative Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus: A Population-Based Cohort Study. J Diabetes Res 2020; 9161039.
- Li R, Ryu C, Munie M. Association of Metformin Treatment with Reduced Severity of Diabetic Retinopathy in Type 2 Diabetic Patients. J Diabetes Res 2018; 2801450.
- Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27: 283–290.
- Adamiec-Mroczek J, Zając-Pytrus H, Misiuk-Hojło M. Caspase-Dependent Apoptosis of Retinal Ganglion Cells during the Development of Diabetic Retinopathy. Adv Clin Exp Med 2015; 24: 531–535.
- Guzman DC, Olguín HJ, García EH. Mechanisms involved in the development of diabetic retinopathy induced by oxidative stress. Redox Rep 2017; 22: 10–16.
- Sun J, Xu Y, Sun S, Sun Y, Wang X. Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: the role of mitochondrial reactive oxygen species. Mol Cell Biochem 2010; 343: 27–35.
- Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 2001; 50: 1938–1942.
- Haskins K, Bradley B, Powers K. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci 2003; 1005: 43–54.
- Kowluru RA, Shan Y. Role of oxidative stress in epigenetic modification of MMP 9 promoter in the development of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2017; 255: 955–962.
- Xiao C, He M, Nan Y. Physiological effects of superoxide dismutase on altered visual function of retinal ganglion cells in db/db mice, PLoS One 2012; 7: e30343.
- Fukumoto M, Nakaizumi A, Zhang T. Vulnerability of the retinal microvasculature to oxidative stress: ion channeled-pendent mechanisms, Am J Physiol Cell Physiol 2012; 302: C1413–C1420.
- Van der Velden JL, Wagner DE, Lahue KG. TGFβ1-induced deposition of provisional extracellular matrix by tracheal basal cells promotes epithelial-to-mesenchymal transition in a c-Jun NH2-terminal kinase-1-dependent manner. Am J Physiol Lung Cell Mol Physiol 2018; 314: L984–L997.
- Lou HD, Wang SY, Guo T, Yang Y. Role of miR-21 in rats with proliferative diabetic retinopathy via TGF-β signaling pathway. Eur Rev Med Pharmacol Sci 2019; 23: 9–16.
- Fan J, Shen W, Lee SR. Targeting the Notch and TGF-β signaling pathways to prevent retinal fibrosis in vitro and in vivo. Theranostics 2020; 10: 7956–7973.
- Chen JL, Luo C, Pu D. Metformin attenuates diabetes-induced tau hyperphosphorylation in vitro and in vivo by enhancing autophagic clearance. Exp Neurol 2019; 311: 44–56.
- Niu C, Chen Z, Kim KT. Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy 2019; 15: 843–870.