ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 16
Chinese herb treats endometritis by regulating inflammatory cytokines and T-cell paradigm in infertility woman
1Department of Reproductive Medicine, Yantai Yuhuangding Hospital, ShanDong University, 44 Wenhua Xi Road, Jinan, Shandong, P.R. China
2Department of Emergency, Yantai Affiliated Hospital of Binzhou Medical College, ShanDong University, 44 Wenhua Xi Road, Jinan, Shandong, P.R. China
3Department of Reproductive Medicine, Yantai Yuhuangding Hospital, Affiliated Hospital of Medical College of Qingdao University, No. 20 Yuhuangding East Road, Yantai, Shandong, P.R. China
- *Corresponding Author:
- Cuifang Hao
Department of Reproductive Medicine
Yantai Yuhuangding Hospital, Affiliated Hospital of Medical College of Qingdao University
Yantai, Shandong, P.R. China
Accepted date: July 24, 2017
Objective: To research that Chinese Herbal Medicine (CHM) can treat Chronic Endometritis (CE) by regulating inflammatory cytokines and T-cell paradigm.
Materials and methods: 16 infertility women were selected in control group, others diagnosed CE were observed in the trial group. The levels of inflammation cytokines (IFNγ, IL-10, IL-17A and TGF-β) were analyzed, both the mRNA and protein expression of T cells subsets specific transcription factors (T-bet, GATA-3, RORγt and Foxp3) were detected.
Result: After CHM treatment, the expression of IFN-γ, IL-17AmRNA were low than the normal value while IL-10, TGF-βmRNA were high, both the mRNA and protein expression of T-bet and RORγt were lower than the healthy whereas GATA-3 and Foxp3 were higher.
Conclusion: CHM is a valid prescription for recommending CE. The mechanism may involve adjusting dynamic equilibrium of pro-inflammatory cytokines, anti-inflammatory cytokines and modulating Th1/ Th2, Th17/Treg paradigm shifting to Th2 and Treg.
Keywords
Chronic endometritis, Inflammatory cytokines, T-cell paradigm, Specific transcription factor, Chinese herbal medicine.
Introduction
Infertility has been becoming the third popular disease in the world for decades [1]. One study showed that Chronic Endometritis (CE) was diagnosed in about one-third of women undergoing hysteroscopy due to reproductive problems [2]. CE refers to a chronic inflammation of the uterus. It usually has no obvious clinical symptoms, and the patients would only feel mild abdominal uncomfortable and have increased leucorrhoea, etc. However, it could impede the pregnancy seriously. It was even reported that 56% of infertile women were diagnosed CE [3]. At present, the diagnosis of CE is generally based upon endometrial biopsies with the presence of plasma cells in the endometrial stroma [4]. The endometrium plasma cells can actively display with the application of CD38, CD138 while they are illegible in HE staining. CE diagnostic parameters is as following: the presence of hyperaemia, mucosal edema and micropolyps (less than 1 mm in size) under the hysteroscopy [2,5].
It is well-known that cytokines can regulate the inflammatory response. When pro-inflammatory cytokines prevail, chronic inflammation develops. However, when anti-inflammatory cytokines and pro-inflammatory cytokines are in the state of homeostasis, inflammation is eliminated. IFN-γ, T-helper 1 (Th1) type cytokines and also a pro-inflammatory cytokine, is detrimental to pregnancy at high concentrations [6]. IL-10, which is mainly, secreted by T-helper 2 (Th2) cells, characterized by anti-inflammatory immune response [7]. At present, two new subsets T-helper 17 (Th17) and regulatory T cells (Tregs) have been discovered. IL-17A is a proinflammatory cytokine mainly expressed by T helper 17 cells [8]. TGF-β, which is secreted by Treg cells, is an antiinflammatory cytokine [9]. Tortorella et al. had discovered that the microenvironment of CE patient changes which is likely to be a reflection of a Th1/Th2 cytokine imbalance (Th1 cytokine dominate) at the endometrial level 3. Therefore, we can infer that it is possible that Th17/Treg cytokine imbalance (Th17 cytokine dominate) is related with CE.
CD4+ T cells, including Th1, Th2, Th17 cells and Treg, play an important role in the fetomaternal immune response [8]. At the maternal-foetal interface, multiple immune mechanisms, including the Th1/Th2 balance, participate in induction of immune tolerance that protects the embryo from maternal attack [10]. The Th17 lineage can cause severe human inflammatory diseases [11]. The activity of Th17 cells is attenuated by the anti-inflammatory action of Tregs [8]. T-bet is a major T cell transcription factor that regulates the expression of Th1 cytokine genes and it determines the development of the Th1 lineage [12,13]. GATA-3 is Th2 specific and up-regulated during Th2 differentiation [14]. RORγt is the specific transcription factor for the differentiation of Th17 cells [15]. Foxp3 is required for Treg cell differentiation and that its high expression is a characteristic feature of Treg cells [16,17]. Accumulated evidences have shown that Th1/Th2, Th17/Treg paradigm shifted into Th2 and Treg would promote pregnancy [18-20]. Based on this, we assume that whether the onset of CE is related to the bias of T cell paradigm, Th1/Th2, Th17/Treg paradigm shifted into Th1 and Th17, then influences pregnancy.
Untreated endometritis can decrease the opportunity of spontaneous pregnancy and assisted reproduction [21]. Antibiotic treatment has been the first choice of drugs for CE, but sometimes there is no significant effect of prolonged or repeated multiple antibiotics combining therapy [22]. Moreover, prolonged antibiotic treatment can cause the body resistance or flora disturbance, leading to other diseases [23]. Traditional Chinese Medicines (TCM) as a system of medicine of China can be traced back over 2500 y ago. Nowadays, lots of Chinese Herbal Medicine (CHM) and extracts have shown various positive treatment effects including bacteriostasis, antiinflammation and anti-cancer [24]. Clinical practice has proved that CHM has a positive therapeutic effect in treating CE.
In our study, CE diagnosed by hysteroscopy and histology was treated with CHM and then compared related indicators to evaluate efficacy. The mRNA expression of pro-inflammatory cytokines (IFN-γ, IL-17A) and anti-inflammatory cytokines (IL-10,TGF-β) was detected. Furthermore, both the mRNA and protein expressions of Th1, Th2, Th17 and Treg specific transcription factors (T-bet, GATA-3, RORγt and Foxp3) were assayed.
Materials and Methods
Subjects and treatment protocols
The study protocol was approved by the Institutional Ethical Review Board of Yuhuangding Hospital of Yantai. Each patient with in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) was examined by hysteroscopy. The evaluation of the endometrial mucosa was performed by the same doctor. The presence of hyperaemia, mucosal edema, and micropolyps (less than 1 mm) were considered as diagnostic criteria. Participants in the trial group were taken curettage. Meanwhile, some patients who had one or more implantation failure or other reasons had curettage. All the endometrial biopsy was examined by pathology.
32 women participated voluntarily for the research in the Department of Reproductive Medicine of Yantai Yuhuangding Hospital from October 2014 to 2015. 32 patients were divided into two groups: group one, 16 infertility women without any identified problems were selected as control group. 16 patients who diagnosed CE by hysteroscopy and histology were assigned in the trial group, and had been treated with CHM. The period of treatment was 3 months. Then the patients in the trial group had another hysteroscopy and were taken curettage as previous described protocol. Assisted pregnancy treatment was carried out in the following month. IVF/ICSI was performed on the patients without frozen embryos and Thaw Embryo Transfer (TET) was done on the ones who had frozen embryos. Patients in control group were given IVF/ICSI or TET treatment after appropriate preparation.
All the women were less than 40 y old. None of them had used hormonal treatment in the following 3 months. Written information was obtained from all the patients before participating in the study. Those patients who had uterine abnormality (septum, unicornuate uterus), submucous myoma, adhesion of uterine cavity, endometriosis and endometrial hyperplasia or tuberculosis by histological diagnosis were excluded.
The Chinese herbal medicine (CHM)
All plants of CHM (Table 1) were genuine Chinese medicine ingredients and purchased from traditional Chinese medicine dispensary, Yantai Yuhuangding Hospital. All participants were taught to boil the herbs by a specialized nurse: Dip the herbs into water for about 30 min.
| Chinese name | English name | Latin name | Famiy | Weight (g) | Part used |
|---|---|---|---|---|---|
| Yimucao | Motherwort | Leonurus japonicus Houtt | Labiatae | 30 | Aerial part |
| Jixueteng | Suberect spatholobus Stem | Caulis spatholobi | Leguminous | 30 | Stem |
| Xianlingpi | Epimedium Herb | Herba epimedii | Berberidaceae | 30 | Leaf |
| Qiancao | India Madder Root | Radix rubiae | Rubiaceae | 15 | Aerial part |
| Danggui | Angelica | Angelica sinensis (Oliv.) Diels | Umbelliferae | 15 | Root |
| Gualou | Fructus trichosanthis | Trichosanthes kirilowii Maxim | Section of the gourd | 15 | Peel |
| Xuduan | Himalayan Teasel Root | Radix dipsaci | Sichuan broken families | 15 | Root |
| Taoren | Peach kernel | Prunus persica (L.) Batsch | Rosaceae | 12 | Seed |
| Chuanniuxi | Radix cyathulae | Cyathula officinalis Kuan | amaranthaceae | 10 | Root |
| Danpi | Tree Peony Bark | Cortex moutan | Ranunculaceae | 10 | Root |
| Rougui | Cinnamon | Cinnamomum verum | Lauraceae | 10 | Bark |
| Fuling | Poria cocos | Poria cocos Schw. Wolf. | Porous bacteria | 10 | Sclerotium |
| Chishao | Radix paeoniae Rubra | Radix Paeoniae Rubra | Ranunculaceae | 10 | Root |
| Xiangfu | Cyperus rotundus | Rhizoma cyperi | Cyperaceae | 10 | Rhizome |
| Wulingzhi | Trogopterus Dung | Faeces trogopterori | Petauristidae | 10 | Faeces |
| Yujin | Curcuma aromatica | Curcuma aromatica Salisb. | Jiang Ke | 10 | Rhizome |
| Ezhu | Zedoary | Rhizoma curcumaewere | Jiang Ke | 10 | Rhizome |
| Zaojiaoci | Chinese Honeylocust Spine | Spina gleditsiae | Leguminous | 10 | Thorn |
| Sanleng | Rhizome of Common Burreed | Sparganii rhizoma | Sparganiaceae | 10 | Rhizome |
Table 1: Composition of Chinese herbal medicine (CHM).
Keep the level of water a little higher than one finger. Then use the boiler (ceramic or stainless steel but avoiding iron) to boil it with high heat for 5 min. After that, keep it boiling with a gentle heat for 25 min. Repeat these steps again. Mix the liquid adequately and cool it to 39-41°C naturally.
The patients bend knees and take left before bed, choose smaller catheter which is coated with liquid paraffin oil, gently insert anus 25-30 cm, enema should not be too much, usually 80-150 ml or so. After clysis they take knee-chest position, in order to extend the enema retention time. Generally speaking, it is 14 d for one treatment course in a menstrual cycle. The treatment was performed in non-menstrual period.
Clinical observation
In order to assess the therapeutic effect of CHM, patients in these groups were observed until we submitted this article. The thickness of Endometrium (EM), the number of high quality embryos which were transplanted in cycles was recorded on the day of embryo transplantation.
The participants were assigned for a second visit in 14 d and a third visit in 40 d after embryo transplantation. They were asked to exam blood β-HCG and vaginal B ultrasound to find out whether pregnancy or not. Clinical pregnancy rate (clinical pregnancy cycles/transfer cycles) and embryo implantation rate (total number of implantation embryos/total number of embryo transfer) were calculated.
Western blot analysis
Proteins were extracted from the samples according to manufacturer’s instructions. The concentration was determined by BCA Protein Assay. Protein samples were separated by Sodium Dodecylsulfate-Polyacrylamide Gel Electrophoresis (SDSHPAGE) (8%). Then they were transferred to polyvinylidene fluoride membrane using a Bio-Rad electro blot apparatus. The membrane was blocked in 7% fat-free milk in TBST, followed by incubation at 4°C overnight with primary antibodies as following: anti-T-bet mouse monoclonal antibody (Abcam Inc., Abcam, Cambridge, USA; 1:250), anti-GATA-3 mouse monoclonal antibody (Abcam Inc., Abcam, Cambridge, USA; 1:1000), anti-RORγt mouse monoclonal antibody (Abcam Inc., Abcam, Cambridge, USA; 1:500) and anti-Foxp3 mouse monoclonal antibody (Abcam Inc., Abcam, Cambridge, USA; 1:1000). After washing with TBST, the membranes were incubated at room temperature for 1 h with secondary antibodies as following: goat peroxidase-conjugated antimouse IgG, (Beyotime Biotechnology, Beyotime, Haimen, China; 1:1000). GAPDH was used as loading control. The protein bands were visualized by chemiluminescence using ECL kit (SupreSignalWest Pico; Thermo Scientific, USA), and the results were analyzed by Image J analysis software to calculate the gray value, the relative intensity of the target protein band was reduced from the ratio to that of the synchronous positive control Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH).
Reverse transcription-polymerase chain reaction
Total RNA was extracted from endometrium using the RNeasy Mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. After the concentration and purity of RNA were evaluated using the ND-1000 NanoDrop spectrophotometer (NanoDrop, Wilmington, DE), the RNA was reversedly transcribed into cDNA using PrimeScript RT Reagent Kit (TaKaRa, Shiga, Japan) in the Rotor-Gene 3000 Real-time PCR system (Corbett Research, Sydney, Australia) with the following parameters: 15 min RT reaction at 37°C, 5 s reverse transcriptase inactivation reaction at 85°C and then quenched at 4°C. Quantitative real time-polymerase chain reaction (qRT-PCR) was conducted to measure the levels of mRNA by using the 10.0 μl SYBR Premix Ex Taq II (TaKaRa, Shiga, Japan), 2 μL total cDNA, each forward and reverse primers 0.8 μL and 20.0 μL total volume following the manufacturer’s protocol. Reactions were performed using the Rotor-Gene 3000 Real-time PCR system (Corbett Research, Australia) with the following reaction profile: pre-denaturation for 30 s at 95°C and PCR amplification for 45 cycles with 5 s at 95°C, 60 s at 60°C. The PCR was followed by a melt curve analysis to determine the reaction specificity. The housekeeping gene Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was used to normalize the expression level because its expression was stable. It is calculated mean ± SD for the change in gene expression in each group as individual data points using the equation 2-ΔΔCt.
The following primers were used to quantify mRNA levels: human T-bet, forward primer 5’-GGC GAA GGA GAC TCT AAG-3’ and reverse primer 5’-ATT AAC ACA CAT GCC AAC GAA-3’; human GATA-3, forward primer 5’-GCC CTA CGT GCC CGA GTA CAG-3’ and reverse primer 5’-GGC CGG GTT AAA CGA GCT G-3’; human RORγt, forward primer 5’-AGC ACA CCG GAG GCA CCC TAT-3’ and reverse primer 5’-CCG GCA CAT CCT AAC CAG-3’; human Foxp3, forward primer 5’-AGT TCC ACA ACA TGC GAC C-3’ and reverse primer 5’-TGA AGT AAT CTG TGC GAG CAG-3’; human IFNγ, forward primer 5’-A TGAAATATACAAGTTATATCTTGGCTTT-3’ and reverse primer 5’-GATGCTCTTCGACCTCGAAACAGCAT-3’; human IL-10, forward primer 5’- TGAATTCCCTGGGTGAGAAG-3’ and reverse primer 5’- CTCTTCACCTGCTCCACTGC-3’; human IL-17A, forward primer 5’-GATGCCCAAATTCTGAGGACAAG-3’ and reverse primer 5’-CCACGGACACCAGTATCTTCTC-3’; human TGF-β, forward primer 5’- TACTAGATTTCGTTGTGGGTTTCC-3’ and reverse primer 5’-TTCGGTCCAGTTGCCTTCTC-3’. GAPDH RNA was quantified as a control to normalize differences in total RNA levels using the following primers: 5’- GGGAAACTGTGGCGTGAT-3’ (forward) and 5’- GAGTGGGTGTCGCTGTTGA-3’ (reverse). Each set of qRTPCR reactions was repeated three times.
Statistical analysis
The Statistical Package for Social Sciences (SPSS, version 16.0, SPSS Inc., and Chicago, IL) for Windows was used. The results were expressed as mean ± standard deviation (SD). The data was analyzed by one-way ANOVA followed by the Least Significant Difference-t (LSD-t) test, the student’s t-test, Wilcoxon rank test and Chi-squared (X2) test were used to compare the categorical variables. Statistical significance was accepted at P<0.05.
Results
Demographic characteristics of subjects
The demographic characteristics of the subjects (no matter in IVF/ICSI cycle or TET cycle) were presented in Table 2. The age, infertile duration, times of previous ET/TET, a comparison between the number of patients with primary infertility or secondary infertility and the number of patients who had frozen embryos were analyzed, and no significant differences were found among the two groups (P>0.05).
| The control group | The trial group | t/X2 | P | |
|---|---|---|---|---|
| Number | 16 | 16 | ||
| Age (y) | 32.06 ± 4.11 | 32.19 ± 3.95 | -0.088 | 0.931 |
| Infertile duration (y) | 3.13 ± 2.39 | 3.56 ± 2.28 | -0.53 | 0.6 |
| Primary infertility (n) | 6 | 8 | 0.508 | 0.722 |
| Secondary | ||||
| Infertility (n) | 10 | 8 | ||
| Times of previous | ||||
| ET/TET (y) | 1.58 ± 1.11 | 1.56 ± 1.12 | 0.035 | 0.973 |
| Number of patients who had frozen embryos (n) | 5 | 7 | 0.533 | 0.465 |
Table 2: Characteristics of subjects.
A comparison on assisted reproductive situation and clinical pregnancy outcomes between the two groups
The number of high-quality embryos and endometrial thickness had no difference between the control group and the trial group (p>0.05) (Table 3). But compared with the control group, embryo implantation rate (38.71% vs. 31.25%) of the trial group after CHM treatment was higher than the control group. Also the clinical pregnancy rate (56.25% vs. 50.00%) of the trial+CHM group was higher, but there was no statistical difference (p>0.05).
| The control group | The trial+CHM group | t/X2/Z | P | |
|---|---|---|---|---|
| Number (n) | 16 | 16 | ||
| Embryo transfer number (n) | 2.00 ± 0.00 | 1.94 ± 0.25 | -1 | 0.317 |
| EM (mm) | 10.69 ± 1.49 | 10.38 ± 1.86 | 0.525 | 0.604 |
| Implantation | 31.25% (10/32 ) | 38.71% (12/31) | 0.386 | 0.535 |
| Rate | ||||
| Clinical pregnancy rate | 50.00% (8/16 ) | 56.25% (9/16 ) | 0.125 | 0.723 |
Table 3: A comparison on assisted reproductive situation and clinical pregnancy outcomes between the two groups.
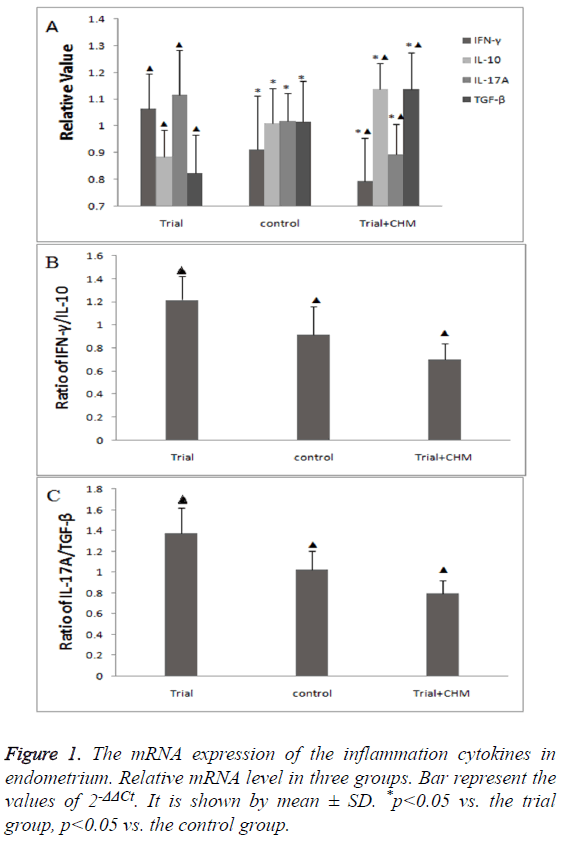
The mRNA expression of inflammation cytokines in endometriumThe mRNA expression of inflammation cytokines in endometrium
It was found that the mRNA expression of pro-inflammation cytokine IFNγ and IL-17A in trial group were both significantly higher than that in the control group. Meanwhile, the mRNA expression of anti-inflammation cytokine IL-10 and TGF-β was much lower in the trial group (p<0.05) (Figure 1A). After CHM treatment, the mRNA expression of IFNγ and IL-17A decreased. On the other hand, the mRNA expression of IL-10 and TGF-β increased considerably after CHM administration (p<0.05) (Figure 1A).
The ratios of Th1 cytokines to Th2 cytokines, Th17 cytokines to Treg cytokines are shown in Figures 1B and 1C. The ratio of IFN-γ to IL-10 showed a significant increasing (p<0.01) in trial group compared to the control group, and significantly decreased (p<0.01) after receiving treatment. In addition, the ratio of IL-17A to TGF-β showed the same trend, the trial group was dramatically higher than the control group (p<0.01). However, after CHM treatment, the ratio dramatically decreased (p<0.01).
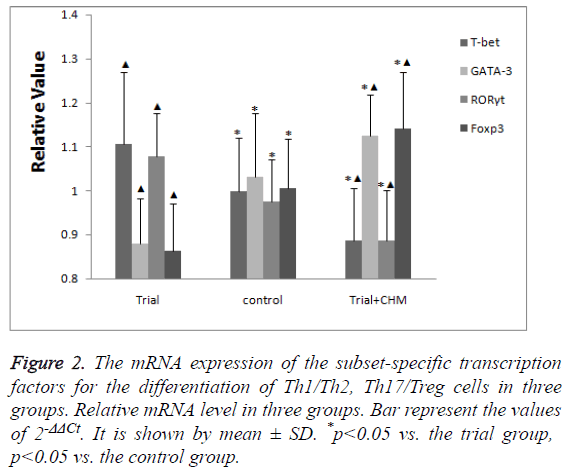
The mRNA expression of subset-specific transcription factors in endometrial CD4+ T cells
Compared with the control group, the mRNA expression of the subset-specific transcription factors for Th1 cells (T-bet) and Th17 cells (RORγt) were high while those for Th2 cells (GATA-3) and Treg (Foxp3) were low in trial group (p<0.05) (Figure 2).
Figure 2: The mRNA expression of the subset-specific transcription factors for the differentiation of Th1/Th2, Th17/Treg cells in three groups. Relative mRNA level in three groups. Bar represent the values of 2-ΔΔCt. It is shown by mean ± SD. *p<0.05 vs. the trial group, p<0.05 vs. the control group.
After the administration of CHM, the mRNA expression of Tbet and RORγt decreased while those of GATA-3 and Foxp3 increased more significantly (p<0.05) (Figure 2).
The data indicated that CHM promoted the mRNA expression of Th2 and Treg specific transcription factors while inhibited those of Th1 and Th17 specific transcription factors.
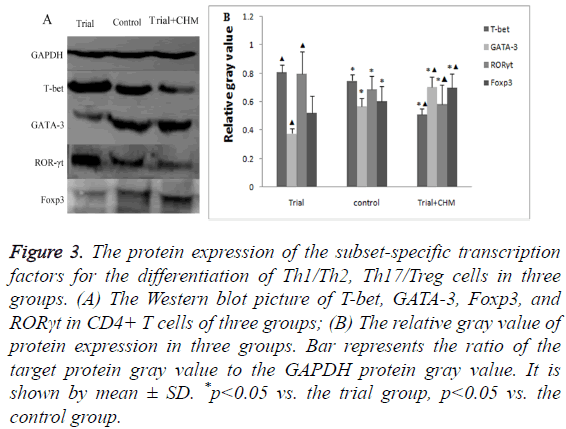
The protein expression of subset-specific transcription factors in endometrial CD4+ T cells
Similar to the mRNA expression changes found in RT-PCR, the protein level of T-bet and RORγt were high whereas those of GATA-3 and Foxp3 were low in trial group (p<0.05) (Figures 3A and 3B). After the administration of CHM, the protein level of T-bet and RORγt decreased while those of GATA-3 and Foxp3 increased (p<0.05) (Figures 3A and 3B). The outcome confirmed that CHM promoted the protein expression of Th2 and Treg specific transcription factors while inhibited those of Th1 and Th17 specific transcription factors.
Figure 3: The protein expression of the subset-specific transcription factors for the differentiation of Th1/Th2, Th17/Treg cells in three groups. (A) The Western blot picture of T-bet, GATA-3, Foxp3, and RORγt in CD4+ T cells of three groups; (B) The relative gray value of protein expression in three groups. Bar represents the ratio of the target protein gray value to the GAPDH protein gray value. It is shown by mean ± SD. *p<0.05 vs. the trial group, p<0.05 vs. the control group.
Discussion
Infertility has been increasing in recent years worldwide and it is even encountered by one in five couples [25,26]. Of all various factors, a functioning and receptive endometrium is crucial for embryo implantation. CE is a persistent inflammation of the endometrial lining. Research confirms that CE is influential to impaired endometrial receptivity, and patients with repeated implantation failure (RIF) often accompany by CE [27]. Although in most cases CE is asymptomatic or accompanied by mild disturbances, it may cause severe reproductive consequences on natural fertility as well as IVF cycles [28].
Although a variety of cells can secrete cytokines, the principal producers are the T helper cells and macrophages [29]. Cytokines can handle the inflammatory response [30]. Inflammation alters endometrial cytokine production, and this may have impaired endometrial functions resulting in reducing embryo receptivity [31]. According to the different role that plays in the process of inflammation, inflammatory cytokines can be divided into two groups: pro-inflammatory cytokines and anti-inflammatory cytokines. The former can promote the development of inflammation while the latter can inhibit the development of inflammation, promote the process of repair and regeneration of tissue. Therefore, whether to keep the balance between the two influences the outcome of inflammation [32]. IFN-γ, a pro-inflammatory cytokine, mainly produced by activated Th1 cells, can suppress the activation and proliferation of Th2 cells. It also suppresses the production of Th2-type cytokines [30]. IL-10 is the most important anti-inflammatory cytokine in human immune responses. It is secreted mainly by Th2 cells, and has antiinflammatory effects through inhibiting of antigen presenting function and reducing Th1 cells to secrete IFN-γ [33]. Th17 cells can produce IL-17A. IL-17A is an important proinflammatory cytokine. It plays an important role in inductingmaintaining chronic inflammatory [34]. TGF-β, which is generated by Treg cells, had been thought of primarily as an anti-inflammatory cytokine [35,36]. Various cytokines coordinate, induce or antagonize, making balance or imbalance in constantly changing. Eventually these exert proinflammatory effects or anti-inflammatory effects. In our study, the expression of pro-inflammatory cytokines IFN-γ, IL-17AmRNA increased in the trial group than the normal group (p<0.05) while the expression of anti-inflammatory cytokine IL-10, TGF-βmRNA decreased (p<0.01), exerting pro-inflammatory effects. Proper cytokine’s production may prepare of the endometrium so that altered levels or function may result in partial, or complete, success or failure of implantation in humans [37]. However, after CHM administration, the expression of IFN-γ, IL-17AmRNA significantly reduced (p<0.01). Rather, IL-10, TGF-βmRNA level were elevated than pre-treatment (p<0.01). Antiinflammatory cytokines restrict pro-inflammatory cytokines, endometrium receptivity was improved. Subsequently clinical pregnancy rate and embryo implantation rate elevated. The trend of ratio of Th1 cytokine to Th2 cytokine, Th17 cytokine to Treg cytokine suggested that it is associated with CE.
The immunologic mechanisms induced by T cells may play an important role in pre-implantation embryo development, in implantation and in the phenomenon of foetal allograft tolerance [38]. Changes in the balance of Th1, Th2, Th17 and Treg cell cytokine profiles may contribute to the failure of implantation of the embryo [8]. It is well known that a strong Th1 environment is harmful to pregnancy and the Th2 response ameliorates the Th1 response [39]. Th1/Th2 balance to maintain a Th2 dominant (anti-inflammatory) environment, leading to maintenance of pregnancy [40]. Once Th1 prevails, it will stimulate the production of pro-inflammatory cytokines and then damage the embryo, consequently, implantation failure or spontaneous abortion will occur [27]. Th17 cells, a distinct subset of CD4+ T-helper cells, are characterized by secretion of IL-17A [41]. Th17 cells are commonly associated with chronic inflammatory and autoimmune diseases. Accumulated evidences have shown that Th17 cells play a deleterious role in the process of pregnancy [42,43]. Tregs are helpful to maintain immunological tolerance and may play a role in the maintenance of pregnancy [9,35]. At the meantime, the activity of Th17 cells is attenuated by the antiinflammatory action of Tregs [8]. Th1/Th2, Th17/Treg paradigm bias to the Th2 and Treg would promote pregnancy [9]. T-bet is a Th1-specific T box transcription factor [44]. GATA-3 is Th2 specific and up-regulated during Th2 differentiation [14]. The orphan nuclear receptor RORγt is the key transcription factor that orchestrates the differentiation of the Th17 lineage while transcription factor Foxp3 has been shown to be associated with the development of Treg [15,35]. In this study, the mRNA and protein expression of Th1 and Th17-specific transcription factors (T-bet and RORγt) increased in the trial group than the control group (p<0.05) while those of Th2 and Treg-specific transcription factors (GATA-3 and Foxp3) decreased (p<0.05). However, after treatment of CHM, both the mRNA and protein expression of Th1 and Th17 specific transcription factors (T-bet and RORγt) were dropped (p<0.05). In the meantime, those of Th2 and Treg-specific transcription factors are (GATA-3 and Foxp3) dramatically raised (p<0.01). This may change the Th2 and Treg environment to promote pregnancy.
TCM has been used in China for 2,500 y. There has no diagnosis about CE in TCM, but it belongs to the category of “leukorrheal diseases”, “infertility”, “woman abdominal pain”, “irregular menstruation”, “woman miscellaneous diseases”. In the TCM theory, CE happens in the period of menstrual and postpartum. When main and collateral channels are empty, chronic diseases involves kidney, causing deficiency of kidney. Or usually physically weak, the evil invades the body, blocking channels, resulting in Qi and blood stagnation. All these factors cause the disease. Its pathological nature is deficiency and excessively intermingling, asthenia of the kidneyQi stagnation and blood stasis. Therefore, treatment principle is invigorating the kidney, promoting Qi and remove blood stasis, quickening blood circulation. In CHM compound, Epimedium Herb (Xianling- pi), Himalayan Teasel Root (Xu-duan), Cinnamon (Rougui) invigorate the kidney Qi, Fructus trichosanthis (Gua-lou), Poria cocos (Fu-ling), Tree Peony Bark (Dan-pi), Trogopterus Dung (Wu-ling-zhi), Curcuma aromatica (Yu-jin) clear away heat and remove dampness, promote flowing of Qi and blood, dissolve the stagnant status; Motherwort (Yi-mu-cao), Suberect spatholobus Stem (Ji-xue-teng), India Madder Root (Qiancao), Angelica (Dang-gui), Cyperus rotundus (Xiang-fu), Peach kernel (Tao-ren), Radix cyathulae (Chuan-niu-xi), Radix paeoniae Rubra (Chi-shao), Zedoary (E-zhu), Rhizome of Common Burreed (San-leng), Chinese Honeylocust Spine (Zao-jiao-ci) promote blood circulation and make main and collateral channels easily and smoothly, eliminate the mass and relieve swelling. The CHM treatment has functions of invigorating the kidney, promoting Qi and removing blood stasis, quickening blood circulation. It can promote the flowing of blood issue, improve the body’s metabolic function, promotes elimination of inflammation. In addition, enema is absorbed from the rectum and acts on the pelvic cavity, relative drug concentration is increased in pelvic cavity, which accelerate blood circulation, relieves vasospasm, then achieve faster and more effective outcome.
All in all, CHM have a good effect on treating CE whether it is diagnosed by histology or hysteroscopy. After CHM treatment, the clinical pregnancy rate and embryo implantation rate of those patients in the trial group were higher than the control group. Patients with CE have immune dysfunction, in endometrium the expression of pro-inflammatory cytokines IFN-γ, IL-17AmRNA increased dramatically while the expression of anti-inflammatory cytokine IL-10, TGF-βmRNA decreased. The ratio of IFN-γ to IL-10IL-17A to TGF-β is associated with CE. Simultaneously, both the mRNA and protein expression of Th1 and Th17-specific transcription factors (T-bet and RORγt) were higher than the normal value whereas Th2 and Treg-specific transcription factors (GATA-3 and Foxp3) were lower than the normal value. However, after CHM administration, the situation is completely opposite to the previous. Based on this, we inferred that the onset of CE is related to the bias of T cell paradigm, Th1/ Th2, Th17/Treg paradigm shifted to Th1 and Th17 is pathological mechanism of CE. CHM can treat CE by reversing T cell subsets offset that Th1/Th2, Th17/Treg paradigm shifted to Th2 and Treg. Next schedule is to do flow cytometry analysis to verify this inference.
Conclusion
The research provides an effective method of treating CE. And it demonstrated that CHM treat CE effectively from immune regulation. However, the effective phytochemical compounds of CHM need further investigation.
Acknowledgment
The authors would like to gratefully thank Ningning Wang for her warm-hearted help in the language revising, thank Zhenteng Liu and Huangguan Dai for technology help.
References
- Peyron R, Aube´ny E, Targosz V. Early termination of pregnancy with mifepristone (RU486) and the orally active prostaglandin misoprostol. New Engl J Med 1993; 328: 1560-1561.
- Cicinelli E, Resta L, Nicoletti R. Detection of chronic endometritis at fluid hysteroscopy. J Minim Invas Gynecol 2005; 12: 514-518.
- Tortorella C, Piazzolla G, Matteo M. Interleukin-6, interleukin-1β, and tumor necrosis factor α in menstrual effluent as biomarkers of chronic endometritis. Fertility Sterility 2014; 101: 242-247.
- Kiviat NB, Wolner-Hanssen P, Eschenbach DA. Endometrial histopathology in patients with culture-proved upper genital tract infection and laparoscopically diagnosed acute salpingitis. Am J Surg Pathol 1990; 14: 167-175.
- Cicinelli E, Resta L, Nicoletti R. Endometrial micropolyps at fluid hysteroscopy suggests the existence of chronic endometritis. Hum Reprod 2005; 20: 1386-1389.
- Qiu L. Study on changes of serum T helper cell type 1 and type 2 cytokines after active immunotherapy in women with unexplained habitual abortion. Zhonghua Fu Chan Ke Za Zhi 2001; 36: 408-410.
- Mor G, Cardenas I, Abrahams V. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann New York Acad Sci 2011; 1221: 80-87.
- Wen-Juan W, Fu-Jun L, Cui-Fang H. Adoptive transfer of pregnancy-induced CD4+CD25+ regulatory T cells reverses the increase in abortion rate caused by interleukin 17 in the CBA/JÃ-BALB/c mouse model. Hum Reprod 2014; 29: 946-952.
- Wu L, Luo L H, Zhang Y X. Alteration of Th17 and Treg cells in patients with unexplained recurrent spontaneous abortion before and after lymphocyte immunization therapy. Reprod Biol Endocrinol Rb E 2014; 12: 74.
- Mingdong Z, Ruijin Z, Xiaoyan X. IL-10 reduces levels of apoptosis in Toxoplasma gondii-infected trophoblasts. Plos One 2013; 8: 331-338.
- Gagliani N, Vesely M C, Iseppon A. Th17 cells trans differentiate into regulatory T cells during resolution of inflammation. Nature 2015; 523.
- Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev 1996; 9: 532-562.
- Szabo SJ, Kim ST, Costa GL. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000; 100: 655-669.
- Zhang DH, Cohn L, Ray P. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem 1997; 272: 21597-21603.
- Ivanov II, Mckenzie BS, Liang Z. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006; 126: 1121-1133.
- Shohei H, Takashi N, Shimon S. Control of regulatory T cell development by the transcription factor Foxp3. Sci 2003; 299: 1057-1061.
- Khattri R, Cox T, Yasayko SA. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunol 2003; 4: 337-342.
- Wilczyński J R, Radwan P, Tchórzewski H. Immunotherapy of patients with recurrent spontaneous miscarriage and idiopathic infertility: does the immunization-dependent Th2 cytokine overbalance really matter? Archivum immunologiae et therapiae experimentalis 2012; 60: 151-160.
- Saifi B, Rezaee SA, Tajik N. Th17 cells and related cytokines in unexplained recurrent spontaneous miscarriage at the implantation window. Reprod Biomed Online 2014; 29: 481-489.
- Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta 2014; 35: 241-248.
- Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol 2006; 194: 630-637.
- Le J, Xing X, Youji F. Obstetrics and gynecology (6th ed). People’s Medical Publishing House, Beijing, China 2004.
- Zhu HR. Clinical and experimental research progress of chronic endometritis treated by Chinese medicine. Shanghai J Trad Chin Med 2007; 41: 75-78.
- Chen G, Yang Y, Liu M. Banxia xiexin decoction protects against dextran sulfate sodium-induced chronic ulcerative colitis in mice. J Ethnopharmacol 2015; 166: 149-156.
- Gao W, Liang JX, Liu S. Oxidative damage of DNA induced by X-irradiation decreases the uterine endometrial receptivity which involves mitochondrial and lysosomal dysfunction. Int J Clin Exp Med 2015; 8: 3401-3410.
- Lessey BA. Assessment of endometrial receptivity. Fertility Sterility 2011; 96: 522-529.
- Yang R, Du X, Wang Y. The hysteroscopy and histological diagnosis and treatment value of chronic endometritis in recurrent implantation failure patients. Archiv Gynecol Obstetr 2014; 289: 1363-1369.
- Cravello L, Porcu G, D’Ercole C. Identification and treatment ofendometritis. Contracept Fertil Sex 1997; 25: 585-586.
- Lohning M, Hutloff A, Kallinich T. Expression of ICOS in vivo defines CD4qeffector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med 2003; 197: 181-193.
- Sukhikh GT, Kasabulatov NM, Van’ko LV. Ratio between the number of Th1 and Th2 lymphocytes in the peripheral blood and concentration of proinflammatory cytokines in lochia of women with postpartum endometritis. Bulletin Exp Biol Med 2005; 140: 672-674.
- Maybin JA, Critchley HO, Jabbour HN. Inflammatory pathways in endometrial disorders. Mol Cell Endocrinol 2011; 335: 42-51.
- Zong H. Experimental study on the effects of activating blood and tonifying the kidney on inflammatory cytokines and adhesion-related indices in the rats with chronic pelvic inflammatory disease. Shandong Univ Trad Chin Med 2010.
- Casatell MA, Meda L, Gasperini S. Interleukin-10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leucocytes by delaying mRNA degradation. J Exp Med 1994; 179: 1695-1699.
- Huang C, Li Y, Fan X. IL-17A promotes pulmonary inflammation in rats with pulmonary fibrosis induced by bleomycin. Chin J Cell Mol Immunol 2014; 30: 366-370.
- Chen WJ, Jin W, Hardegen N. Conversion of peripheral CD4+ CD25- naive T cells to CD4+ CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 2003; 198: 1875-1886.
- Fantini MC, Becker C, Monteleone G. Cutting edge: TGF-β induces a regulatory phenotype in CD4+ CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 2004; 172: 5149-5153.
- Pietro C, Cicinelli E, Guglielmino MR. Altered transcriptional regulation of cytokines, growth factors, and apoptotic proteins in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol 2013; 69: 509-517.
- Piccinni MP. T-cell cytokines in pregnancy. Am J Reprod Immunol 2002; 47: 289-294.
- Van NALV, Heineman MJ, Faas MM. The immunology of successful pregnancy. Hum Reprod Update 2003; 9: 347-357.
- Lee JY, Lee M, Lee SK. Role of endometrial immune cells in implantation. Clin Exp Reprod Med 2011; 38: 119-125.
- Samuel H, Nicola G, Flavell R A. Life, death, and miracles: Th17 cells in the intestine. Eur J Immunol 2012; 42: 2238-2245.
- Wang WJ, Hao CF, Yi-Lin. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and deciduas in unexplained recurrent spontaneous abortion patients. J Reprod Immunol 2010; 84: 164-170.
- Liu YS, Wu L, Tong XH. Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol 2011; 65: 503-511.
- Szabo SJ, Kim ST, Costa GL. Pillars article: A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000; 100: 655-669.