ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 15
Changes in the size of breast lesions during menstrual cycle observed by ultrasound: An initial study
1Abant Izzet Baysal University Hospital, Department of Radiology, Bolu, Turkey
2Antalya Ataturk State Hospital, Clinic of Radiology, Antalya, Turkey
- *Corresponding Author:
- Emine Dagistan
Department of Radiology
Abant Izzet Baysal University Hospital
Bolu, Turkey
Accepted date: April 29, 2017
Purpose: We aimed to evaluate changes in size of breast lesions during menstrual phases.
Material and Methods: In this prospective study, 46 women aged 18-35 years old who were referred to our radiology clinic for breast sonography and had cystic or solid breast lesions larger than 5 mm were enrolled to the study. Breast ultrasound (US) was performed at two different times; one within 5 days before and one within 5 days after menstrual bleeding. Anteroposterior and transverse length of the lesions were measured.
Results: In total, 145 breast lesions were detected by US. Of these lesions, 6 were visualized in premenstrual phase but were not observed in postmenstrual phase. 6 lesions different were not visualized in premenstrual, but were detected in postmenstrual phase. One hundred and twenty-three lesions were visualized by US both in luteal and follicular phases.
Conclusions: Timing of breast US might cause false results and unnecessary further investigations. Therefore, we suggest that breast US in women should be performed in follicular phase of the menstrual cycle.
Keywords
Breast lesion, Ultrasonography, Menstrual cycle.
Introduction
Ultrasound scan (US) is widely used in the evaluation of breast parenchyma and breast lesions. It is useful in the definition of the lesions detected clinically or by mammography (MG) or magnetic resonance imaging (MRI). It is also important in the differentiation of benign and malignant breast lesions and the radiologic follow up of the breast lesions. In addition, it is the primary imaging modality of the breast during pregnancy and lactation [1]. Although MG is an efficient screening test for postmenopausal women, it has reduced sensitivity in younger women due to dense breast parenchyma. In such cases US is used as a supplementary imaging modality to increase accuracy rates [2].
Hormonal fluctuations during luteal phase of menstrual cycle affect breast parenchyma by stimulating proliferation and edema. It can be seen as an increase in density of parenchyma in mammography which results in a decrease in the sensitivity of this imaging modality in detecting breast lesions [3]. Moreover, these menstrual changes may cause more contrast enhancement in breast tissue during MRI. Thus, detection of the lesions becomes hard and false positive results may increase. Therefore, MRI is suggested to be performed in the first half of menstrual cycle, especially within 7 days after menstrual bleeding stopped [4]. To date, changes in the ultrasonographic characteristics of breast lesions during menstrual cycle have not been studied. In the present study, we aimed to observe sonographic changes in breast lesions during menstrual phases.
Materials and Methods
This prospective study was approved by the Local Ethics Committee for Clinical and Laboratory Studies and conducted in accordance with the latest version of Helsinki Declaration. Also, it was supported by local Scientific Research Administration of our institution. All patients provided written informed consent.
Study population
From January 2014 to July 2015, women aged 18-35years old who were referred to our US clinic for breast sonography was enrolled to the study. Patients were selected with the following inclusion criteria: (a) regular menstruation at least for last six months; (b) normal menstrual cycle length (21-35 days) [5], (c) menstrual bleeding lasting 3 to 7 days; and (d) having cystic breast lesions larger than 5 mm in diameter or solid breast lesions.
Exclusion criteria were as follows: (a) current pregnancy; (b) current breastfeeding or breastfeeding within last 3 months; (c) using oral contraceptives or hormone replacement therapy; (d) having menstrual irregularity; (e) hormonal diseases such as polycystic ovary syndrome; (e) receiving chemotherapy or radiotherapy within 1 year, (f) previous breast surgery involving the quadrant of detected lesion.
After informed consent and enrolling to the study, inventories were completed for all patients evaluated in the study, that included a) demographic data, b) menstrual cycle characteristics (cycle length, bleed length), c) medical history, d) sonographic findings of breast lesions.
Radiologic assessment
Breast US was performed at two different times; one within 5 days before and one within 5 days after menstrual bleeding. The first 15 participants were examined by two radiologists (one 4 years and other 9 years experienced) at the same time in sake of obtaining a common vision and technique. Following participants after first 15 scanned by either of the two radiologists, but, pre and postmenstrual US performed by the same radiologist in each woman. An US device eligible for harmonic observation was used for imaging studies General Electric LOGIQ S8 (General Electric Medical Systems, Milwaukee, WI, US). A linear US probe with 6-15MHz was used for the study protocol. Space-occupying lesions in US examination were grouped into four categories; simple cysts, complicated cysts (low-level internal echoes or intracystic debris), complex cysts (thick walls, thick septa, intracystic masses, or other discrete solid components) and solid lesions. Anteroposterior and transverse length of the lesions were measured before and after the menstrual bleeding. The sizes of the lesions were calculated by average of anteroposterior and transverse diameters. The changes in the size of the lesions in premenstrual phase compared to postmenstrual phase were recorded. Small simple cysts (smaller than 5 mm) were not enrolled to the study. The largest five cysts were mentioned during the study in case of having multiple (more than 5) simple cysts, but, all other space-occupying lesions (complicated cysts, complex cysts, solid lesions) included in the study. Additionally, the quadrant of the lesions and the distance from the nipple line were recorded.
Statistical analysis
SPSS software (SPSS 15.0 for Windows, IBM, Chicago, IL, USA) used for statistical analysis. Characteristics of the patients and the lesions were demonstrated as mean ± standard deviation or number (%). The dimensions of the lesions were checked for normal distribution with the Kolmogorov-Smirnov one-sample test for goodness of fit. None of them were normally distributed; therefore, the nonparametric Wilcoxon signed-rank test was used to compare the measurements before and after menstrual bleeding. Data are presented as the median (25th percentile-75th percentile). We utilized Pearson’s r method (dividing the z-score by the square root of the sample size) to determine the effect size.
Results
A total of 46 women met the inclusion criteria. In total, 145 breast lesions were detected by US. Of these lesions, 6 were visualized in premenstrual phase but were not observed in postmenstrual phase on US. While different 6 lesions were not visualized in premenstrual phase, but were detected in postmenstrual US. One hundred and twenty-three lesions were visualized both in luteal and follicular phase US. In addition, 5 women had more than 5 breast lesions (7-23 lesions). We assumed these lesions shall have similar menstrual pattern, therefore 5 largest lesions were selected for those women. Remaining 107 lesions were analyzed statistically. Table 1 shows the characteristics of the study population.
| Mean ± SD (range) | Number (%) | ||
|---|---|---|---|
| Age (years) | 27.7 ± 6.1 (18-35) | ||
| Body mass index | 21.7 ± 3.5 (16.1-34.5) | ||
| The number of the lesions | 3.1 ± 3.9 (1-23) | ||
| Duration of the menstrual cycle (days) | 28.5 ± 3.3 (21-35) | ||
| Duration of the bleeding (days) | 5.8 ± 1.4 (3-7) | ||
| Marital status | Single | 29 (63%) | |
| Married | 17 (37%) | ||
| Parity | Nulliparous | 31 (67.3%) | |
| Parous | 15 (32.6%) | ||
| Number of births | Two | 10 (66.6%) | |
| One | 5 (33.3%) | ||
| Breastfeeding history | No | 31 (67.3%) | |
| Yes | 15 (32.6%) | ||
| Mastodynia | No | 24 (52.2%) | |
| Yes | 22 (47.8%) | ||
| Cumulative duration of breastfeeding (months) | 22.1 ± 11.3 (4-42) | ||
Table 1: Characteristics of the patients.
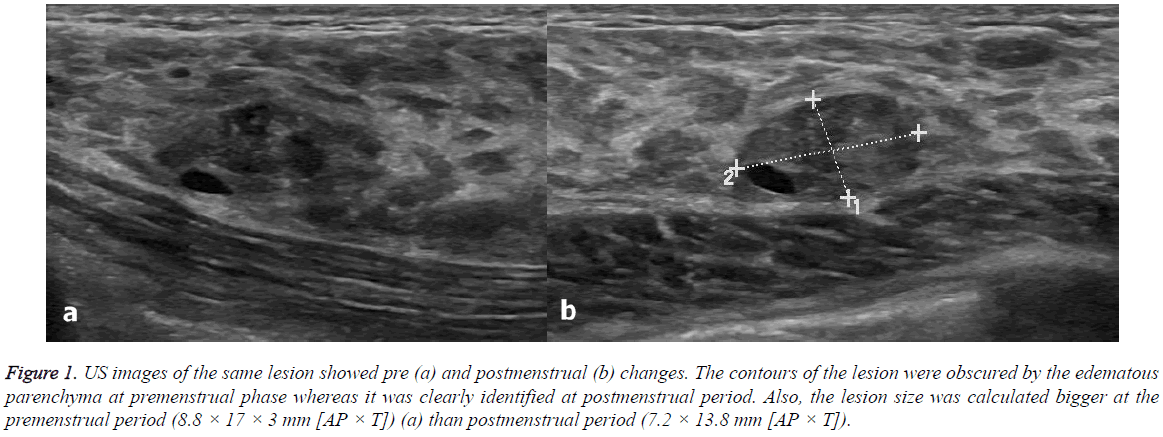
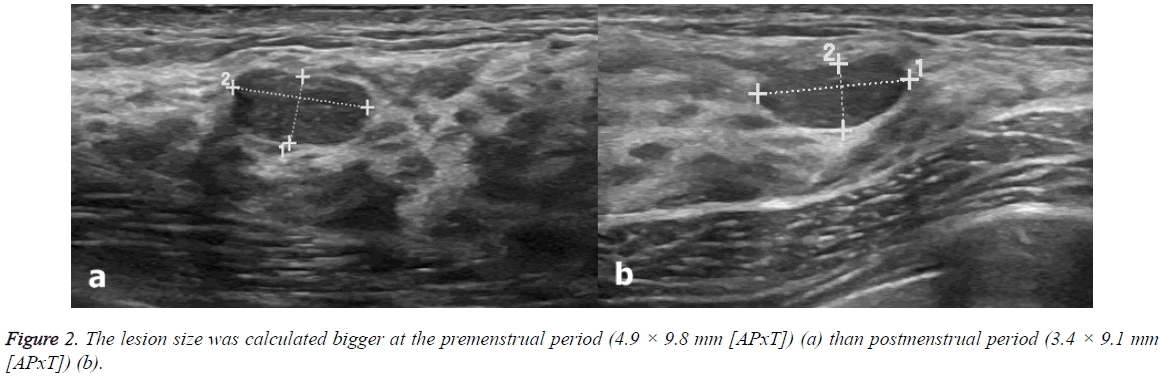
US scans were performed 2.63 ± 1.3 (range: 1-5) days before and 2.5 ± 1.4 (range: 1-5) days after menstrual bleeding (Figures 1 and 2). None of the participants had been using oral contraceptives. Only two patients had been taking chronic medications (both were using thyroid hormone replacement therapy for hypothyroidism). Table 2 shows the characteristics of the breast lesions and Table 3 shows the changes in the size of the lesions before and after menstrual bleeding. Dimensions of the lesions significantly reduced in follicular phase US (after menstrual bleeding) compared to those in luteal phase (before menstrual bleeding) (p<0.05). Significant change in the diameter of the lesions in postmenstrual period compared to premenstrual period observed.
Figure 1: US images of the same lesion showed pre (a) and postmenstrual (b) changes. The contours of the lesion were obscured by the edematous parenchyma at premenstrual phase whereas it was clearly identified at postmenstrual period. Also, the lesion size was calculated bigger at the premenstrual period (8.8 × 17 × 3 mm [AP × T]) (a) than postmenstrual period (7.2 × 13.8 mm [AP × T]).
| n (%) | ||
|---|---|---|
| Location | Right | 55 (51.4%) |
| Left | 52 (48.6 %) | |
| Quadrant | Upper lateral | 69 (65.5%) |
| Lower lateral | 19 (17.8%) | |
| Lower medial | 7 (6.5%) | |
| Upper medial | 12 (11.2%) | |
| Lesion type | Simple cyst | 9 (8.4%) |
| Complicated cyst | 14 (13.1%) | |
| Complex cyst | 2 (1.9%) | |
| Solid lesion | 82 (76.6%) | |
Table 2: Characteristics of the lesions.
| mm | Median (25th-75th percentiles) | Z | Effect size | P value |
|---|---|---|---|---|
| Premens AP | 5.4 (4-8.8) | -6.823 | 0.631 | 0.003 |
| Postmens AP | 5.1 (3.7-8.1) | |||
| Premens T | 8 (6-11,7) | -6.997 | 1.545 | <0.001 |
| Postmens T | 7,6 (5.7-10.1) |
AP: Anteroposterior Length, T: Transverse Length
Table 3: Changes in size of the lesions before and after menstrual bleeding*.
Additionally, the authors observed that the borderline of the lesions were identified clearly in follicular phase compared to luteal phase.
Discussion
We found in the present study that there were significant changes in the sonographic characteristics of the breast lesions of women according to menstrual phases. To our knowledge, this is the first study in the literature reporting such variations in the size of breast lesions in luteal and follicular phases.
The normal breast undergoes changes through the menstrual cycle because of hormone sensitive nature. Ramakrishna et al. have histo-pathologically confirmed that breast tissue have larger lobules, increased stromal edema and mixed inflammatory infiltrates during the second half of menstrual cycle, namely luteal phase [6]. Increased parenchymal volume of the breasts in this phase has been shown in two MRI studies [7,8]. Similarly, using mammography, White et al. have shown that there was a significant variation of breast density by time in the menstrual cycle; which was highest in luteal phase [9].
Underlying mechanism of cyclic changes in breast US may be associated with the increased density of breast tissue in luteal phase. Histopathologic studies have reported greater epithelial cell proliferation, lobule size and stromal edema in luteal phase compared to follicular phase [10,11]. Fluid component of the breast tissue may influence the image quality obtained by US, which is sensitive to the water compound of the soft tissues.
US is the first diagnostic approach of breast lesions in women younger than 40 years old [12]. Younger women are more likely to have dense breast tissue than the older women [13,14]. Increased density may decrease the likelihood of detection of the lesions in breast US. Increased breast density may reduce the sensitivity of mammography and cause false results [15,16]. Similar challenges have been encountered in breast MRI by variation of the tissue enhancement during menstrual cycle [4,17,18]. During the luteal phase, contrast enhancement of normal breast tissue might mimic breast lesions and cause false-positive results. Additionally, background parenchymal enhancement might hide a small malignant lesion leading to false-negative results. According the findings of these studies, performing breast MRI in follicular phase was suggested to minimize these effects [4,17,18].
Previous studies differentiated breast lesions by contrast enhanced MRI [19]. As discussed above, we have similar concerns about the timing of breast US as in breast MRI and MG. The results of present study showed that these concerns may be justified. In the present study, the sizes of the lesions were calculated larger in luteal phase compared to follicular phase. Also, the authors have observed that the breast parenchyma obscured the borderline of the lesions in luteal phase causing difficulty in measuring the size of the lesions. Breast US is the principal imaging technique in young adult women [20]. False positive or negative results would lead misdiagnosis or unnecessary further imaging techniques such as MG and MRI, which are more harmful or much more expensive, compared to US. The results of a surveillance cohort study by Kuhl et al. have showed that US detected only 17 of 40 breast cancer cases [21]. The authors did not mention the timing of US in their study, however, we think that luteal phase US may be the underlying cause of misdiagnosis of the cases.
We assume that, according to the present results, detection of the breast lesions by US is more likely in follicular phase of the menstrual cycle. However, present study has some limitations to point out: (a) self-report of the participants in defining the menstrual cycle, (b) not mentioning the body mass index which could influence breast density and (c) relatively small sample size which challenge our results to interpret.
Conclusion
Timing of breast US might cause false results and unnecessary further investigations. For this reason, we suggest that breast US in women should be performed in follicular phase of the menstrual cycle.
References
- Bassett LW, Kimme-Smith C. Breast sonography. AJR Am J Roentgenol 1991; 156: 449-455.
- Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology 1995; 196: 123-134.
- Ursin G, Parisky YR, Pike MC, Spicer DV. Mammographic density changes during the menstrual cycle. Cancer Epidemiol Biomarkers Prev 2001; 10: 141-142.
- Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Garrido L. Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. The breast J 2005; 11: 236-241.
- Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. Journal of obstetric, gynecologic, and neonatal nursing. JOGNN 2006; 35: 376-384.
- Ramakrishnan R, Khan SA, Badve S. Morphological changes in breast tissue with menstrual cycle. Modern pathology 2002; 15: 1348-1356.
- Fowler PA, Casey CE, Cameron GG, Foster MA, Knight CH. Cyclic changes in composition and volume of the breast during the menstrual cycle, measured by magnetic resonance imaging. BJOG 1990; 97: 595-602.
- Graham S, Stanchev P, Lloyd‐Smith J, Bronskill M, Plewes D. Changes in fibroglandular volume and water content of breast tissue during the menstrual cycle observed by MR imaging at 1.5 T. J Magn Reson Imaging 1995; 5: 695-701.
- White E, Velentgas P, Mandelson MT, Lehman CD, Elmore JG, Porter P, Yasui Y, Taplin SH. Variation in mammographic breast density by time in menstrual cycle among women aged 40–49 years. J Natl Cancer Inst 1998; 90: 906-910.
- Longacre TA, Bartow SA. A correlative morphologic study of human breast and endometrium in the menstrual cycle. Am J Surg Pathol 1986; 10: 382-393.
- Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens progestogens normal breast cell proliferation and breast cancer risk. Epidemiol Rev 1993; 15: 17-35.
- Mendelson E, Baum J, Berg W, Merritt C, Rubin E. Breast imaging reporting and data system: ACR BI-RADS-breast imaging atlas. BI-RADS: Ultrasound Reston. Am College of Radiol 2003.
- Oza AM, Boyd NF. Mammographic parenchymal patterns: a marker of breast cancer risk. Epidemiol Rev 1992; 15: 196-208.
- Gram I, Funkhouser E, Tabar L. Reproductive and menstrual factors in relation to mammographic parenchymal patterns among perimenopausal women. Br J Cancer 1995; 71: 647.
- Ma L, Fishell E, Wright B, Hanna W, Allan S, Boyd N. Case-control study of factors associated with failure to detect breast cancer by mammography. J Natl Cancer Inst 1992; 84: 781-785.
- Bird RE, Wallace TW, Yankaskas BC. Analysis of cancers missed at screening mammography. Radiol 1992; 184: 613-617.
- Müller-Schimpfle M, Ohmenhaüser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiol 1997; 203: 145-149.
- Ellis RL. Optimal timing of breast MRI examinations for premenopausal women who do not have a normal menstrual cycle. AJR Am J Roentgenol 2009; 193: 1738-1740.
- Uematsu T, Kasami M. MR imaging findings of benign and malignant circumscribed breast masses: part 1. Solid circumscribed masses. Jpn J Radiol 2009; 27: 395-404.
- D'Orsi CJ, Mendelson EB, Ikeda DM. ACR breast imaging and reporting data system: breast imaging atlas. Am Coll of Radiol 2003.
- Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005; 23: 8469-8476.