ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
Beneficial effect of Mangiferin against sleep deprivation-induced neurodegeneration and memory impairment in mice
Department of Paediatrics, Shaanxi Province, Yanan University Hospital, Shaanxi, China
- *Corresponding Author:
- Kun-Xia Xie
Department of Paediatrics
Shaanxi Province, Yanan University Hospital
Yanan, P.R. China
Accepted date: June 23, 2016
Pre-clinical and clinical studies have suggested that sleep loss is a predisposing factor for neurodegeneration and memory impairments linked with Alzheimer’s disease (AD) and about 45% of AD patients show sleep disturbances. Sleep deprivation (SD) alters immuno-inflammatory, oxidantantioxidant enzymes balance and brain derived neurotrophic factor (BDNF) pathways which play significant role in the neurodegeneration and cognitive impairments associated with AD. Mangiferin (MGF), a C-glucosylxanthone, possesses multiple activities together with antioxidant, antiinflammatory, anti-anxiety, antidepressant and neuroprotection. The current study evaluated the beneficial effect of MGF (40 mg/kg, p.o.) and Piracetam (200 mg/kg, p.o.) pre-treatment (14 days) on chronic (5 days) SD-induced memory impairment, inflammation and BDNF depletion in both hippocampus (HC) and plasma as well as hippocampal oxidative damage in mice. MGF and Piracetam pre-treatment significantly improved the memory impairment by reducing escape latency while increasing number of crossings over platform position in Morris water maze (MWM) test. Recognition index also improved without affecting total exploratory time in novel object recognition (NOR) test by MGF and Piracetam pre-treatment. MGF and Piracetam pre-treatment attenuated SD-induced neurodegeneration by reducing inflammatory cytokines (IL-1β, TNF-α and IL-6) in both plasma and HC as well as hippocampal oxidative stress via decreasing lipid peroxidation along with restoration of glutathione level in HC. Chronic pre-treatment of MGF and Piracetam also prevented the SD evoked decrease in plasma and hippocampal BDNF level in mice. In conclusion, findings of the present study suggests that MGF showed significant beneficial effect against SD-induced neurodegeneration and memory impairments which may be attributed to its inhibitory action on pro-inflammatory cytokines, oxidative damage and depletion of BDNF level in both plasma and HC. These findings support the potential applicability of MGF in the management of neurodegeneration and memory impairments often seen in neurological disorders linked with inflammation, oxidative stress and BDNF pathway.
Keywords
Inflammation, Memory impairment, Neurodegeneration, BDNF, Sleep deprivation
Introduction
Alzheimer’s disease (AD) is a non-curable, multifactorial, persistent, progressive age-related neurodegenerative disorder commonly occurs in elderly characterized by neuronal loss, cognitive dysfunction, loss of memory and other neurobehavioral symptoms [1,2]. Globally, it is the main cause of dementia and 4th leading cause of the death in the developed countries after cancer, cardiovascular diseases and stroke [3]. It is the major health burden for many countries due to lack of effective treatment or preventive therapy. Currently, cholinergic and glutamatergic systems based drugs are employed for AD treatment but these provides indicative relief from behavioural and cognitive manifestations without affecting its advancement. Therefore, there is utmost need to search novel, effective and safe therapeutic and preventive agents. However, cholinergic therapy is successful in the treatment of AD which pushes us to think about behavioural aspects of AD as a target for the development of better therapy [3].
Sleep plays significant role in the synaptic plasticity and neuronal revival process which are important for the brain’s normal function and performance [4,5]. It is also necessary for the growth and overall development of the living organisms and performs roles such as brain temperature regulation, detoxification process in neuron, energy preservation, tissue repair, immune response and synaptic plasticity [6-11]. Evidences suggest that accumulation of β-amyloid (Aβ) peptides in the brain tissues causes neuronal cell damage leading to neurodegeneration which further develops AD [12]. Numerous pathways such as inflammatory, BDNF signalling, oxido-nitrosative stress, DNA damage repair and autophagy are involved in the neurodegeneration which is governed by the biological clock [13,14]. The early signs of neurodegenerative disorders including Alzheimer’s are alterations in quality and pattern of sleep, and circadian rhythms which worsen with disease progression as well as aging [15-17]. Sleep is important because it clears Aβ peptides accumulated during daytime while wakefulness increases Aβ concentration in brain tissues. Moreover, good night’s sleep plays neuroprotective function in human beings [18,19].
There are ample of evidences which support that sleep deprivation induces cognitive dysfunction in humans and animals. Clinical studies suggested that sleep deprivation is a predisposing factor for the pathogenesis of AD and about 45% of AD patients show sleep disturbances [1]. Chronic sleep deprivation diminishes hippocampal activity and increases deposition of Aβ and plague, inhibits hippocampal cell proliferation and neurogenesis leading to memory impairments and ultimately development of AD [5,18].
Accumulating evidences have suggested that inflammatory cytokines, oxidative damage and brain derived neurotrophic factor (BDNF) play significant role in the neurodegeneration and cognitive impairments associated with AD [20,21]. Previous studies have suggested that inflammatory response is directly linked to the neurodegeneration associated with AD. Neuroinflammation is one of the hallmarks of AD and inflammatory mediators such as cytokines can be important biomarkers for the early screening and diagnosis of AD [22]. Numerous studies demonstrated the increased IL-1β, TNF-α and IL-6 level in blood and hippocampus of AD patients [21,23]. Immuno-inflammatory pathway activated due to neurodegeneration, Aβ and neurofibrillary tangles [21]. Both pre-clinical and human studies have established that peripheral inflammatory response affects memory functions due to increased pro-inflammatory cytokines in the blood [23]. Moreover, peripheral and central pro-inflammatory cytokines influence each other release in the brain and blood, leading to cascade of inflammatory processes [3,22,23]. Studies have reported that sleep deprivation increases the release of pro-inflammatory cytokines i.e. IL-1β, TNF-α and IL-6 by activating reactive oxygen species (ROS) and decreasing antioxidant enzyme (superoxide dismutase) activity in the hippocampus tissues [3]. BDNF may also involve in the inflammatory process [24]. Moreover, oxidative stress and inflammatory pathway attenuate the BDNF secretion in the hippocampus [3]. Chronic sleep deprivation (5 days) increases IL-6 level when compared with control individuals [3], and IL-6 injection given at low dose leads to alteration in non-rapid eye movement (NREM) and decrease in rapid eye movement (REM) sleep [25].
One of the primary reasons of age-related neurodegenerative disorders like AD is lipids, protein and nucleic acid damage induced by oxyradical [26]. Numerous mechanisms such as release of ROS, genetic factors, inflammatory processes and neurotoxicity are the predisposing factors for the oxidative damage [2]. Sleep clear out free radicals generated during wakeful condition while sleep deprivation alters antioxidant defense mechanism and activates the release of ROS which thereby induces neurodegeneration [11,27]. These ROS and altered BDNF level due to sleep deprivation induces oxidative damage in the different parts of the brain notably in hippocampus. Furthermore, this oxidative damage in various parts of the brain alters long-term potentiation (LTP) results in memory impairments [14].
Brain-derived neurotrophic factor (BDNF), a neuroprotective factor, plays significant role in neuronal growth and survival, synaptic plasticity, synaptogenesis, long-term potentiation (LTP) and memory function [28-30]. It is extensively distributed in the discrete brain areas including hippocampus and cortex and involved in the memory acquisition and consolidation [24]. Thus, changes in the BDNF amount or BDNF-TrkB signalling pathway may lead to cognitive dysfunction and memory loss [31]. Preclinical and clinical studies have indicated the role of BDNF in the pathophysiology of cognitive impairment in AD [20]. Studies have reported that plasma and cerebrospinal fluid BDNF level is decreased in age-related cognitive decline patients as well as AD patients [32-34]. Conversely, administration of BDNF prevents neuronal damage and cognitive dysfunctions due to excessive production and accumulation of Aβ in AD [24]. BDNF levels improved in the hippocampus after learning and memory retrieval process.
Multiple lines of evidence indicated that BDNF level is decreased in discrete brain areas such as brain stem, hippocampus and cerebellum as well as in blood plasma and serum after sleep deprivation [3]. There is impairment in the hippocampal ability in behavioural tests including Morris water maze and Novel object recognition due to alteration or lack of BDNF related gene expression in the hippocampus. This indicates the involvement of sleep in the secretion of BDNF. Thus sleep deprivation negatively affect memory function since BDNF involved in the learning and memory processes [35]. These evidences suggest that BDNF plays significant role in the pathophysiology of AD and may be a potential target for AD therapy [24].
Sleep influences various predisposing factors such as oxidative stress, neurogenesis, excitotoxicity, inflammation and Aβ, which are involved in the pathogenesis of AD [3]. Insomnia may be the profound diagnostic feature for AD and treatment of insomnia should be considered while developing or choosing therapeutic options for AD. Moreover, both central and peripheral pro-inflammatory cytokines and BDNF level could be important biomarkers for initial screening and diagnosis of AD.
Mangiferin, a natural C-glucosylxanthone, is an active phytoconstituent obtained from root, bark and leaves of Mangifera indica [36]. It possesses several pharmacological properties like, antioxidant, analgesic, antidiabetic, cardio protective, hepatoprotective, antitumor, immunomodulatory, and anti-inflammatory, anti-anxiety and antidepressant [36-42]. It also offers neuroprotective action in restraint stress induced neuronal damage in rodents by preventing neuroinflammation and oxidative stress in brain [43]. Till date, no study has been conducted to examine the effect of Mangiferin against chronic sleep deprivation-induced neurodegeneration and AD in mice by modulating BDNF, oxidative stress and pro-inflammatory cytokines level in hippocampus and plasma. Therefore, the current study was performed to evaluate the beneficial effect of Mangiferin against sleep deprivation-induced memory impairment in mice and possible role of oxidative stress, inflammation and BDNF.
Materials and Methods
Animals
Swiss albino male mice (weighing 20-25 g) were used for the present study. Mice were housed under standard housing conditions with 12:12 hour light/dark cycle in cages. Standard food pellet and water were provided ad libitum. Mice were acquainted to housing and laboratory conditions for a week prior to experiment. The female mice were not considered because of their hormonal influence on cognitive behaviour of the animal. National Research Council Guide for the Care and Use of Laboratory Animals was followed in all experimental procedure. Animals and all experimental procedure were approved by the Ethics Committee of the institution.
Experimental design and drug treatment
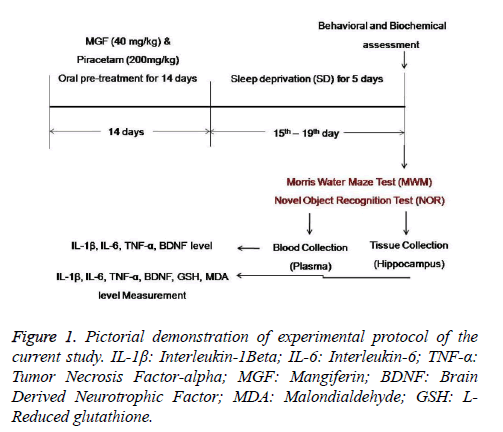
On the day of experiments, mice were randomly divided into 5 groups (n=10) each for behavioural and biochemical estimation as shown in Figure 1.
Group I was treated orally with vehicle i.e. 30% DMSO for 14 days. This group served as Normal control group.
Group II was treated with orally vehicle i.e. 30% DMSO for 14 days and then subjected to sleep deprivation (SD) for 5 days from 15th to 19th day. This group served as Negative control (SD control) group.
Group III was treated with Mangiferin (40 mg/kg, BW, p.o.) for 14 days.
Group IV was treated with Mangiferin (40 mg/kg, BW, p.o.) for 14 days and subjected to chronic SD protocol (5 days) from 15th to 19th day.
Group V was pre-treated with Piracetam (200 mg/kg, BW, p.o) for 14 days and the subjected to SD for 5 days from 15th day to 19th day. This group served as Positive control group.
Before carrying out behavioural studies, animals were acquainted to the laboratory conditions for 1 week duration. All experimental groups were pre-treated with respective drug and dosage for two weeks and then subjected to chronic sleep deprivation protocol for 5 days. Vehicle (30% DMSO), test drug (Mangiferin) [36] and standard drug (Piracetam 200 mg/kg, BW) [44] were given per-orally at dose level of 10 ml/kg and last dose was given 1 h before the behavioural trainings and tests. Memory function was assessed after 24 h of sleep deprivation by subjecting animals different behavioural testes such as Morris water maze (MWM) and novel object recognition (NOR) test. Following behavioural study, mice were sacrificed and brains were dissected out to isolate hippocampus for the measurement of pro-inflammatory cytokines (IL-β, IL-6, TNF-α), BDNF, Malondialdehyde (MDA) and reduced glutathione (GSH) level. Hippocampal homogenates were prepared in ice-cold phosphate buffer (pH 7.4), centrifuged and supernatants were stored at -800ºc until analysis done. Plasma samples were separated from blood and used for the measurement of pro-inflammatory cytokines and BDNF level.
Sleep deprivation method
Sleep deprivation protocol was adapted from the multiple platform method described previously [45]. Briefly, multiple platform method consists of a small platform (3 cm) in a water maze (41 × 34 × 16.5 cm) filled with water. The height of water was kept 1 cm below the platform and bright light was provided whole the night. Animals were subjected to 5 days sleep deprivation protocol by keeping them on small platform. Mice are able to move from one platform to other by jumping. A 100-W light was used to illuminate the chamber during the sleep deprivation period. The principle of this method is that sleep and drowsiness induce muscle relaxation and mice will fall in water, and after falling in water animals will wake up quickly.
Behavioural paradigms
Morris water maze (MWM) test: MWM test is employed to evaluate learning and memory function in the rodents [46]. Water maze test was conducted as per the method described by the Morris [47] with some modifications. Briefly, the apparatus consists of a circular tank filled with water to a depth of 20 cm with temperature 25ºC. The tank was divided into four equal quadrants and a platform (19 cm height) was placed in the centre of the water tank. The position of the platform remained same throughout the training session. Each animal was given 2-4 trails per day for 4-5 days. The animals were released into the water tank and allowed to explore the hidden platform, and 90 sec cut off time was fixed. After 24 h of last training session, animals were subjected to probe trail of 120 sec without platform. Parameters including latency to reach the escape platform and number of crossing over the platform during test was recorded [48].
Novel object recognition test (NORT): NORT was employed to evaluate the recognition memory according to the method explained by Kasbe et al. [46] with some modifications. Briefly, three sessions namely habituation, familiarization, test were employed in NORT. An apparatus made up of wooden box (36 × 50 × 36 cm) with an easy to remove and clean grid floor was used in this test. In habituation phase, each animal was placed in an empty open field area and allowed to explore for 5 min twice in a day to get habituated. After 3 days of habituation session, familiarization phase was done by placing two objects with different shapes (pyramid of 8 cm side and cylinder of 8 cm height) were kept at diagonally opposite corners of the box and animals were allowed to explore freely for 10 min.
In test session, one of the objects used in familiarization phase was replaced with new object (a plastic box), and mice were allowed to explore the box for 5 min. Exploration time with new (N) and familiar (F) objects were recorded separately. Open field arena and objects were cleaned with 70% alcohol between the each session and animals to avoid olfactory cues. Recognition index, a ratio of the exploration time with novel object and total exploration time with both the objects during the test session was recorded and calculated. The results were presented as percentage recognition index [49].
Biochemical analysis
Preparation of hippocampal homogenate: Following behavioural studies, mice were sacrificed by cervical dislocation and brains were immediately removed, hippocampus was dissected out and homogenized in 10% w/v 0.1 M phosphate buffer (pH 7.4). The resultant homogenate were then centrifuged and supernatants were used for neurochemical analysis.
Estimation of hippocampal pro-inflammatory cytokines and BDNF level: Pro-inflammatory cytokines including L-1β, IL-6 and TNF-α were estimated in the hippocampus by using ELISA kits (Diaclone Research, China). The level of these cytokines in 100 μL samples was analysed as per the manufacturer's protocol and results were expressed as pg/ml. Estimation of hippocampal BDNF level was also done by ELISA using BDNF assay kit (Promega, Madison, WI, USA) and BDNF content was expressed as pg/mg of protein.
Estimation of plasma pro-inflammatory cytokines and BDNF level: After behavioural testing, blood was collected from cardiac puncture in chilled heparinized centrifuge tubes and centrifuged at 3000 xg at 4ºC for 15 min. Thus, the obtained plasma was used for the quantification of IL-1β, IL-6, TNF-α and BDNF level by using commercially available ELISA kits for these pro-inflammatory cytokines and BDNF (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Estimation of hippocampal oxidative stress parameters: Lipid peroxidation and reduced glutathione (GSH) assay were done to measure oxidative damage in the hippocampus (HC). Thiobarbituric-acid reacting substances (TBARS) in the form of Malondialdehyde (MDA) level in HC as an index of ROS production was analysed according to the description of Sulakhiya et al. [49]. Lipid peroxidation was analysed by measuring the content of MDA formed by the reaction with thiobarbituric acid at 532 nm spectrophotometrically and expressed as μM of MDA/mg of protein.
Reduced glutathione level in HC was measured as per the procedure of Sulakhiya et al. [50]. The content of GSH measured at 412 nm by using UV-Visible Spectrophotometer, and expressed as μM of GSH/mg of protein. The amount of protein in HC was determined as per Lowry et al. [51] method using bovine serum albumin as standard.
Statistical analysis
Data are presented as mean ± S.E.M. and statistical analysis were done by using One Way Analysis of Variance (ANOVA) followed by Students-Newman-Keuls post hoc test when ANOVA was significant. The results were considered significant at P ≤ 0.05. The GraphPad Prism 5.0 Version for Windows, GraphPad Software (San Diego, California, USA) was used for statistical analysis.
Results
Effect of Mangiferin pre-treatment on SD-induced memory impairments in mice tested in Morris water maze test
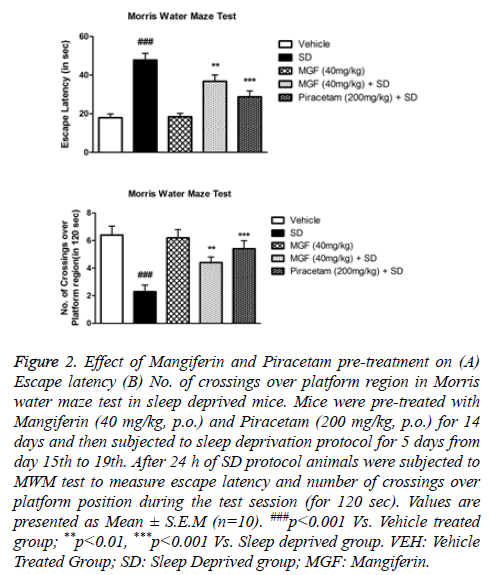
Figure 2 shows that SD induced memory impairments as evident by significant (P<0.001) increase in escape latency (EL) and decrease in number of crossings over platform position in Morris Water Maze test when compared with the vehicle treated mice. Chronic pre-treatment of MGF and Piracetam significantly improved the SD-induced memory impairments in mice. Results show that MGF (P<0.01) and Piracetam (P<0.001) were able to prevent increased escape latency in mice subjected to SD protocol (Figure 2A). Moreover, MGF (P<0.01) and Piracetam (P<0.001) pre-treatment increased the no.of crossings over platform position significantly as compared to sleep deprived animals as shown in Figure 2B.
Figure 2: Effect of Mangiferin and Piracetam pre-treatment on (A) Escape latency (B) No. of crossings over platform region in Morris water maze test in sleep deprived mice. Mice were pre-treated with Mangiferin (40 mg/kg, p.o.) and Piracetam (200 mg/kg, p.o.) for 14 days and then subjected to sleep deprivation protocol for 5 days from day 15th to 19th. After 24 h of SD protocol animals were subjected to MWM test to measure escape latency and number of crossings over platform position during the test session (for 120 sec). Values are presented as Mean ± S.E.M (n=10). ###p<0.001 Vs. Vehicle treated group; **p<0.01, ***p<0.001 Vs. Sleep deprived group. VEH: Vehicle Treated Group; SD: Sleep Deprived group; MGF: Mangiferin.
Effect of Mangiferin pre-treatment on SD-induced recognition memory in mice tested in novel object recognition test
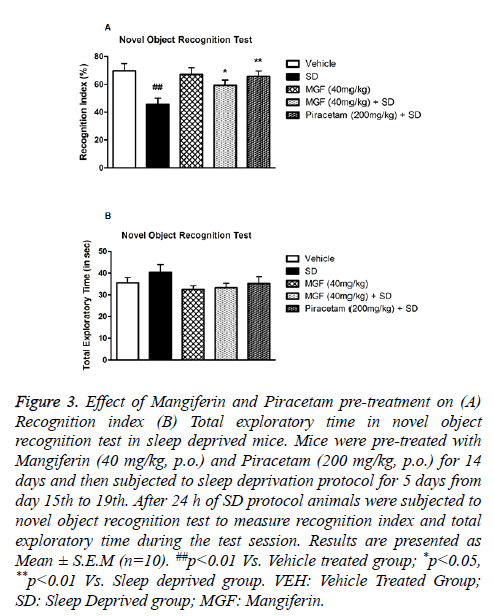
Novel Object Recognition test was performed to investigate the effect of SD on recognition memory in mice. There was significant (P<0.01) decrease in exploratory liking toward novel object in case of sleep deprived (SD) mice when compared with vehicle treated mice. On the other hand, chronic pre-treatment with MGF (P<0.05) and Piracetam (P<0.01) prevented the reduction in recognition index in SD group significantly as shown in Figure 3A. However, total exploratory time of test duration did not alter due to the treatment of MGF and Pircetam, and SD protocol as well as shown in Figure 3B.
Figure 3: Effect of Mangiferin and Piracetam pre-treatment on (A) Recognition index (B) Total exploratory time in novel object recognition test in sleep deprived mice. Mice were pre-treated with Mangiferin (40 mg/kg, p.o.) and Piracetam (200 mg/kg, p.o.) for 14 days and then subjected to sleep deprivation protocol for 5 days from day 15th to 19th. After 24 h of SD protocol animals were subjected to novel object recognition test to measure recognition index and total exploratory time during the test session. Results are presented as Mean ± S.E.M (n=10). ##p<0.01 Vs. Vehicle treated group; *p<0.05, **p<0.01 Vs. Sleep deprived group. VEH: Vehicle Treated Group; SD: Sleep Deprived group; MGF: Mangiferin.
Effect of Mangiferin pre-treatment on SD-induced alterations in hippocampal pro-inflammatory cytokines and BDNF level mice
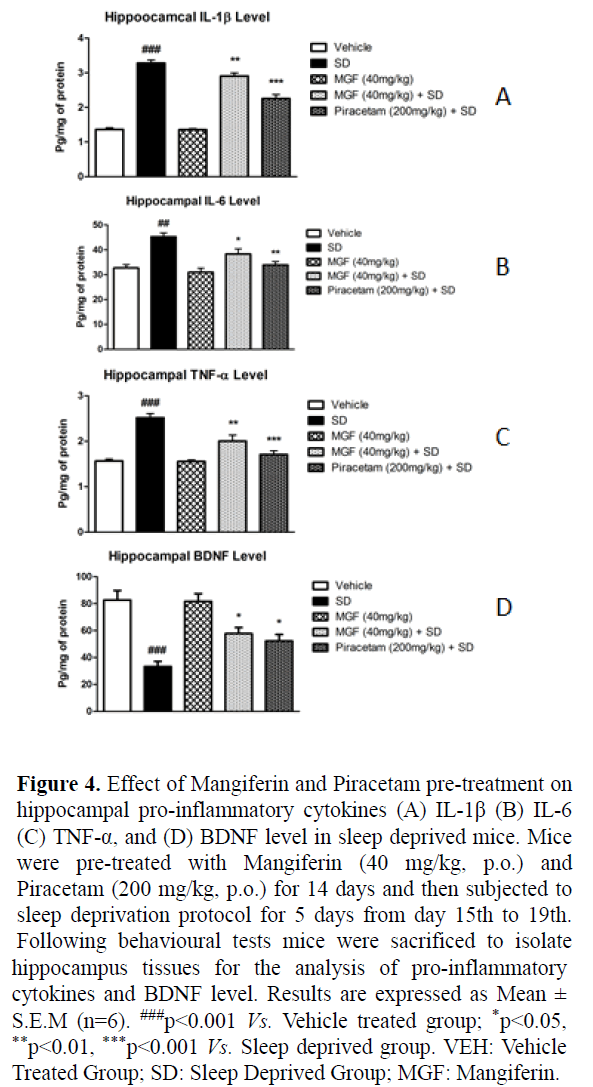
Figures 4A-4C shows that chronic SD induced significant increase in the hippocampal pro-inflammatory cytokines levels including IL-1β (P<0.001), IL-6 (P<0.01), TNF-α (P<0.001), when compared with vehicle treated animals. Fourteen days chronic pre-treatment of MGF significantly attenuated SD-induced increase in hippocampal IL-1β (P<0.01), IL-6 (P<0.05), TNF-α (P<0.01) level when compared with SD group. Moreover, pre-treatment of Piracetam also showed significant protective effect against SD-induced increase in IL-1β (P<0.001), IL-6 (P<0.01), TNF-α (P<0.001) level in hippocampus as compared to SD mice.
Figure 4: Effect of Mangiferin and Piracetam pre-treatment on hippocampal pro-inflammatory cytokines (A) IL-1β (B) IL-6 (C) TNF-α, and (D) BDNF level in sleep deprived mice. Mice were pre-treated with Mangiferin (40 mg/kg, p.o.) and Piracetam (200 mg/kg, p.o.) for 14 days and then subjected to sleep deprivation protocol for 5 days from day 15th to 19th. Following behavioural tests mice were sacrificed to isolate hippocampus tissues for the analysis of pro-inflammatory cytokines and BDNF level. Results are expressed as Mean ± S.E.M (n=6). ###p<0.001 Vs. Vehicle treated group; *p<0.05, **p<0.01, ***p<0.001 Vs. Sleep deprived group. VEH: Vehicle Treated Group; SD: Sleep Deprived Group; MGF: Mangiferin.
In addition, sleep deprivation significantly (P<0.001) decreased BDNF level in the hippocampus of mice when compared with vehicle treated group after 5 days of chronic sleep deprivation whereas significant (P<0.05) improvement in the hippocampal BDNF level was seen in MGF and Piracetam pre-treated animals as shown in Figure 4D.
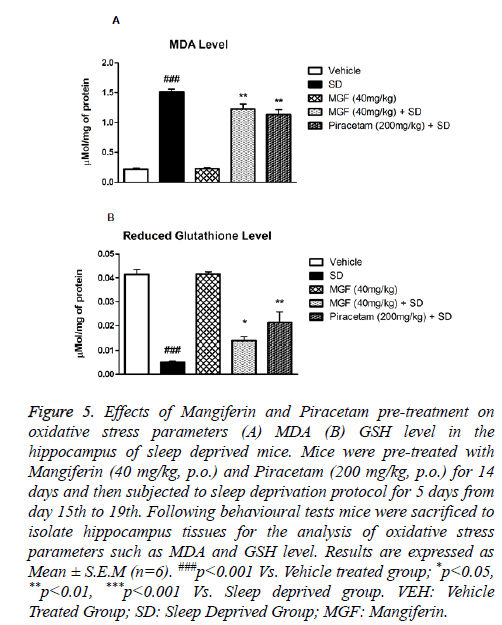
Effect of Mangiferin pre-treatment on SD-induced oxidative stress in hippocampus
Chronic sleep deprived mice showed marked increase in MDA level (P<0.001) in the hippocampus in comparison with vehicle treated mice. Two weeks pre-treatment of MGF and Piracetam significantly (P<0.01) attenuated the increased MDA level as compared to sleep deprived mice as shown in Figure 5A.
Figure 5: Effects of Mangiferin and Piracetam pre-treatment on oxidative stress parameters (A) MDA (B) GSH level in the hippocampus of sleep deprived mice. Mice were pre-treated with Mangiferin (40 mg/kg, p.o.) and Piracetam (200 mg/kg, p.o.) for 14 days and then subjected to sleep deprivation protocol for 5 days from day 15th to 19th. Following behavioural tests mice were sacrificed to isolate hippocampus tissues for the analysis of oxidative stress parameters such as MDA and GSH level. Results are expressed as Mean ± S.E.M (n=6). ###p<0.001 Vs. Vehicle treated group; *p<0.05, **p<0.01, ***p<0.001 Vs. Sleep deprived group. VEH: Vehicle Treated Group; SD: Sleep Deprived Group; MGF: Mangiferin.
There was significant (P<0.001) decrease in the hippocampal reduced glutathione level in mice 5 days post-SD protocol. Both MGF and Piracetam pre-treatment exhibited significant (P<0.01) improvement in GSH level when compared with sleep deprived mice as shown in Figure 5B.
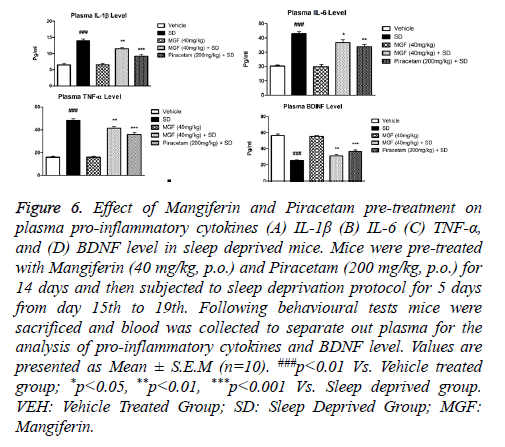
Effect of Mangiferin pre-treatment on SD-induced alterations in plasma pro-inflammatory cytokines and BDNF level mice
Figures 6A-6D show effects of MGF and Piracetam on regulating plasma pro-inflammatory cytokines and BDNF level in a different group of animals. Results showed that 5 days SD induced significant rise in the plasma levels of proinflammatory cytokines IL-1β as shown in Figure 6A: P<0.001; IL-6 as shown in Figure 6B: P<0.001, TNF-α as shown in Figure 6C: P<0.001 and BDNF as shown in Figure 6D: P<0.001 as compared with the vehicle treated group. MGF pre-treatment significantly attenuated plasma levels of various pro-inflammatory cytokines (IL-1β: P<0.01; IL-6: P<0.05, TNF-α: P<0.01) in comparison with vehicle treated mice. Furthermore, pre-treatment with Piracetam also showed significant protection against SD-induced increase in plasma levels of pro-inflammatory cytokines (IL-1β: P<0.001; IL-6: P<0.01, TNF-α: P<0.001).
Figure 6: Effect of Mangiferin and Piracetam pre-treatment on plasma pro-inflammatory cytokines (A) IL-1β (B) IL-6 (C) TNF-α, and (D) BDNF level in sleep deprived mice. Mice were pre-treated with Mangiferin (40 mg/kg, p.o.) and Piracetam (200 mg/kg, p.o.) for 14 days and then subjected to sleep deprivation protocol for 5 days from day 15th to 19th. Following behavioural tests mice were sacrificed and blood was collected to separate out plasma for the analysis of pro-inflammatory cytokines and BDNF level. Values are presented as Mean ± S.E.M (n=10). ###p<0.01 Vs. Vehicle treated group; *p<0.05, **p<0.01, ***p<0.001 Vs. Sleep deprived group. VEH: Vehicle Treated Group; SD: Sleep Deprived Group; MGF: Mangiferin.
In addition, SD group also showed significant decrease in the plasma BDNF level (P<0.001) which was significantly restored by two weeks of chronic pre-treatment of MGF (P<0.01) and Piracetam (P<0.001) when compared with SD mice.
Discussion
Alzheimer’s disease (AD) is a chronic age-related neurodegenerative disorders commonly occur in elderly characterized by memory loss, cognitive deficits and other neurobehavioral manifestations [1,2]. Insomnia is a prominent predisposing factor in the pathogenesis of age-associated neurodegenerative disorders such as Alzheimer’s disease which worsens as the age advances [1,15]. Therefore, it is important to measure neurobehavioral and biochemical effects observed in sleep deprivation animal model [1].
The current study is the first of its kind to disclose that Mangiferin, an antioxidant and anti-inflammatory agents, was able to prevent SD-induced behavioural and neurochemical changes. The results demonstrate that sleep deprivation in mice induces significant learning and cognitive deficit when tested in Morris water maze and novel object recognition test. Moreover, Mangiferin provide protection against SD-induced neurobehavioral and neurochemical changes in mice. In addition, MGF also provide beneficial effect against the rise in pro-inflammatory cytokines levels in the periphery and brain, oxidative stress and restores the decreased level of BDNF both in plasma and hippocampus.
Immuno-inflammatory, BDNF signalling, oxido-nitrosative stress, DNA damage repair and autophagy are the pathways involved in the neurodegeneration which is regulated by the circadian clock [13,14]. Numerous studies suggested that sleep deprivation is a culprit event for the memory impairments and neurochemical changes associated with AD in humans and animals [1]. Furthermore, chronic sleep deprivation provokes deposition of Aβ and plague, inhibits hippocampal cell proliferation and neurogenesis results in neurodegeneration and memory impairments leading to AD [5,18]. Earlier findings have indicated that both acute (24-72 h) and chronic (8 h for 8 weeks) sleep deprivation impairs hippocampus dependent short and long term memory [28]. Results of the present study depict that chronic pre-treatment of MGF significantly attenuated SD-induced memory impairments as observed in different behavioural paradigms. These findings are in accordance with earlier one which demonstrated that chronic administration of MGF prevents cognitive impairments in AlCl3 induced neurotoxicity in mice via inhibiting neuroinflammation and oxidative stress [46].
Inflammation plays significant role in the pathophysiology of AD via neurodegeneration. Neuronal damage, amyloid proteins and neurofibrillary tangles are the common sources of inflammation [3]. Neuroinflammation might increase the peripheral cytokines by reaching out from CNS; on the other hand, peripheral inflammation might be responsible for the neuroinflammation, thus both processes are vise-versa to each other [21]. Preclinical and clinical studies have been suggested that various cytokines i.e. IL-1β, IL-6 and TNF-α elevated in serum which affect cognitive functions in AD patients [23]. The findings of the current study exhibited that chronic sleep deprivation significantly increased the IL-1β, IL-6 and TNF-α level in both plasma and hippocampus of mice which was attenuated by the chronic pre-treatment of Mangiferin and Piracetam. Previous studies have demonstrated that Mangiferin rectify behavioural abnormalities by diminishing proinflammatory cytokines level in both the plasma and hippocampus of the rodents [36]. Moreover, the observed effects may be attributed to antioxidant and anti-inflammatory activity of MGF [36,46].
Oxidative stress plays significant role in memory loss often seen in several health problems including Alzheimer’s disease [26]. There are several reports supporting oxidative stress as one of the factor accountable for the memory impairment in Alzheimer disease via decreasing antioxidant enzymes level in the hippocampus [46]. Sleep deprivation induces ROS generation and alters BDNF level that further causes neurodegeneration through oxidative damage cascade [14]. Current findings indicate that chronic sleep deprivation provokes oxidative damage by increasing MDA and decreasing reduced glutathione level in the hippocampus perhaps responsible for both short- and long-term memory impairments. These previous studies collectively with current results further strongly hold up the concept that oxidative stress plays significant role in the cellular damage and resulting memory impairments. In the current study, Mangiferin provide beneficial effect against SD-induced oxidative damage in the hippocampus by maintaining the balance between oxidant and antioxidant enzyme system via decreasing MDA and increasing GSH level. These findings are in connection with the earlier studies which demonstrated the free radical scavenging activity of Mangiferin. Furthermore, Mangiferin also fight against oxidative stress by maintaining the antioxidant enzyme level such as reduced glutathione in the hippocampus [36,46].
Various studies indicated that BDNF is also have accountability in the pathophysiology of Alzheimer’s disease [3,35]. Sleep regulates the secretion of BDNF which plays significant role in the memory and cognitive function [3,20,35]. Studies indicated that sleep deprivation reduces the BDNF level in discrete brain areas, CSF and serum in AD patients [32-34]. Thus BDNF is important for the memory and cognitive performance of the brain. In the current study, chronic sleep deprivation induced significant decrease in both plasma and hippocampal BDNF level in mice. It may be due to the excessive production of free radicals leading to oxidative hurt of the neurons [3]. These present results are in line with earlier findings that chronic sleep deprivation provoked oxidative stress and neuronal damage thereby decreasing the BDNF level [24,31-34]. Chronic pre-treatment of Mangiferin offered significant improvement against SD-induced decrease in BDNF level in plasma and hippocampus and it may be attributed to its free radical scavenging and anti-inflammatory potential. These findings support the previous results that Mangiferin up-regulated the hippocampal BDNF level in LPS-induced depression [36] and AlCl3 -induced neurotoxicity mouse model [46].
Conclusion
In conclusion, the current study suggested that chronic sleep deprivation induced significant memory impairments and neurochemical alterations in both plasma and hippocampus that leads to neurodegenerative disorder, Alzheimer’s disease. However, two weeks pre-treatment of Mangiferin and Piracetam showed beneficial effect against SD-induced neurodegeneration and memory impairments in mice. This beneficial effect of Mangiferin may be due to inhibition of peripheral and central inflammation, oxidative stress as well as prevention of BDNF depletion in plasma and hippocampus. These findings suggest that Mangiferin may be beneficial agent for the management of neurodegeneration and memory impairments linked with Immuno-inflammatory, oxidative stress and BDNF signalling pathway.
Acknowledgement
The authors are thankful to the Department of Paediatrics, Yanan University Hospital for providing necessary facilities to carry out this study. The authors did not receive any research grant to perform the present study.
References
- Rahman H, Muralidharan P, Sivaraman D, Saha D. Continuous sleep deprivation for 5 days produces loss of memory in mice and may be a cause of Alzheimers disease. Annals Biol Res 2010; 1: 185-193.
- Cardinali D, Furio A, Brusco L. Clinical aspects of melatonin intervention in Alzheimers disease progression. Curr Neuropharmacol 2010; 8: 218-227.
- Sharma VK, Sharma P, Deshmukh R, Singh R. Age Associated Sleep Loss: A Trigger For Alzheimers Disease. Bulletin of Clin Psychopharmacol 2015; 25: 78-88.
- Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Prog Neurobiol 2003; 69: 71-101.
- Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev 2009; 13: 187-194.
- McGinty D, Szymusiak R. Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep. Trends in Neurosci 1990; 13: 480-487.
- Inoue S, Honda K, Komoda Y. Sleep as neuronal detoxification and restitution. Behav Brain Res 1995; 69: 91-96.
- Berger RJ, Phillips NH. Energy conservation and sleep. Behav Brain Res 1995; 69: 65-73.
- Adam K, Oswald I. Sleep is for tissue restoration. J R Coll Physicians of Lond 1977; 11: 376-388.
- Everson CA. Sustained sleep deprivation impairs host defense. Am J Physiol 1993; 265: 1148-1154.
- Maquet P. The role of sleep in learning and memory. Science 2001; 294: 1048-1052.
- Kontush A. Amyloid-β: an antioxidant that becomes a pro-oxidant and critically contributes to Alzheimers disease. F Radi Biol Med 2001; 31: 1120-1131.
- Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci 2012; 13: 325-335.
- Zhang L, Zhang HQ, Liang XY, Zhang HF, Zhang T, Liu FE. Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: role of oxidative stress, BDNF and CaMKII. Behav Brain Res 2013; 256: 72-81.
- Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosoma Res 2004; 56: 497-502.
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 2010; 11: 589-599.
- Van Dongen HPA, Kerkhof GA. Sleep-wake changes and cognition in neurodegenerative disease. Human Sleep and Cognition. Clin Appl Res 2011; 190: 21.
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimers disease pathology. Sci Transl Med 2012; 4: 150.
- Mongrain V, Hernandez SA, Pradervand S. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep 2010; 2: 33.
- Budni J, Bellettini T, Mina F, Garcez ML, Zugno AI. The involvement of BDNF, NGF and GDNF in aging and Alzheimers disease. Aging and disease 2015; 6: 331.
- Bermejo P, Martin S, Benedi J, Susin C, Felici E, Gil P. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimers disease. Immunol Lett 2008; 117; 198-202.
- Delaby C, Gabelle A, Blum D, Schraen S, Moulinier A, Boulanghien J. Central nervous system and peripheral inflammatory processes in Alzheimers disease: biomarker profiling approach. Front Neurol 2015; 6: 181.
- Khemka VK, Ganguly A, Bagchi D, Ghosh A, Bir A, Biswas A. Raised Serum Proinflammatory Cytokines in Alzheimers Disease with Depression. Aging Dis 2014; 5: 170-176.
- Song JH, Yu JT, Tan L. Brain-Derived Neurotrophic Factor in Alzheimers Disease: Risk, Mechanisms, and Therapy. Mol Neurobiol 2014; 52: 1-17.
- Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab 2010; 24: 775-784.
- Deshmukh R, Sharma V, Mehan S, Sharma N, Bedi KL. Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine - a PDE1 inhibitor. Eur J Pharmacol 2009; 620: 49-56.
- Fukui K, Takatsu H, Koike T, Urano S. Hydrogen peroxide induces neurite degeneration: Prevention by tocotrienols. Free Radic Res 2011; 45: 681-691.
- Alzoubi KH, Khabour OF, Rashid BA, Damaj IM, Salah HA. The neuroprotective effect of vitamin E on chronic sleep deprivation-induced memory impairment: the role of oxidative stress. Behav Brain Res 2012; 226: 205-210.
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimers disease. Nat Med. 2009; 15: 331-337.
- Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA. Sleep deprivation prevents stimulation-induced increases of levels of P-CREB and BDNF: protection by caffeine. Mol Cell Neurosci 2011; 46: 742-751.
- Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol 2013; 71: 55-61.
- Li Y, Ji YJ, Jiang H, Liu DX, Zhang Q, Fan SJ. Effects of unpredictable chronic stress on behavior and brain-derived neurotrophic factor expression in CA3 subfield and dentate gyrus of the hippocampus in different aged rats. Chin Med J 2009; 122: 1564-1569.
- Lee JG, Shin BS, You YS, Kim JE, Yoon SW, Jeon DW. Decreased serum brain-derived neurotrophic factor levels in elderly korean with dementia. Psychiatry Investig 2009; 6: 299-305.
- Chakraborty A, Panja S, Bhattacharjee I, Chandra G, Hati A. A longitudinal study of neurocysticercosis through CT scan of the brain. Asian Pac J Trop Dis 2011; 1: 206-208.
- Torabi M, Nasehie M, Zarrindasta MR. Sleep loss and the brain vulnerability to neurodegeneration: behavioral, biochemical and neuro-histopathological observations in a rat model. Excli Journal 2013; 12: 347-372.
- Jangra A, Lukhi MM, Sulakhiya K, Baruah CC, Lahkar M. Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur J Pharmacol 2014; 740: 337-345.
- Garrido G, Gonzilez D, Delporte C, Backhouse N, Quintero G, Nanez AJ. Analgesic and anti-inflammatory effects of Mangifera indica L extract. Phytother Res 2001; 15: 18-21.
- Prabhu S, Narayan S, Devi CS. Mechanism of protective action of mangiferin on suppression of inflammatory response and lysosomal instability in rat model of myocardial infarction. Phytother Res 2009; 23: 756-760.
- Mirza RH, Chi N, Chi Y. Therapeutic potential of the natural product mangiferin in metabolic syndrome. J Nutritional Therapeutics 2013; 2: 74-79.
- Guha S, Ghosal S, Chattopadhyay U. Antitumor, immunomodulatory and anti-HIV effect of Mangiferin, a naturally occurring glucosylxanthone. Chemotherapy 1996; 42: 443-451.
- Hou J, Zheng D, Zhong G, Hu Y. Mangiferin mitigates diabetic cardiomyopathy in streptozotocin-diabetic rats. Canadian J Physiol Pharmacol 2013; 91: 759-763.
- Rao VS, Carvalho AC, Trevisan MTS, Andrade GM, Nobre HV, Moraes MO. Mangiferin ameliorates 6-hydroxydopamineinduced cytotoxicity and oxidative stress in ketamine model of schizophrenia. Pharmacol Reports 2012; 64: 848-856.
- Marquez L, Garca-Bueno B, Madrigal J, Leza JC. Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur J Nutr 2012; 51: 729-739.
- Rahman H, Muralidharan P. Nardostacys jatamansi DC protects from the loss of memory and cognition deficits in sleep deprived Alzheimers disease (AD) mice model. Intr J Pharm Sci Rev Res 2010; 5: 160-167.
- Rahman H, Sindhura S, Eswaraiah MC, Elumalai A. Evaluation of sleep deprivation (a novel Alzheimers disease model) by comparative study with scopolamine and diazepam induced amnesia in mice. Intr J Pharm Sci Rev Res 2011; 11: 5.
- Kasbe P, Jangra A, Lahkar M. Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J Trace Elem Med Biol 2015; 31: 107-112.
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosc Methods 1984; 11: 47-60.
- Singh N, Parle M. Sildenafil improves acquisition and retention of memory in mice. Indian J Physiol Pharmacol 2003; 47: 318-324.
- Palchykova S, Winsky-Sommerer Rl, Meerlo P, DR, Tobler I. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem 2006; 85: 263-271.
- Sulakhiya K, Kumar P, Gurjar SS, Barua CC, Hazarika NK. Beneficial effect of honokiol on lipopolysaccharide induced anxiety-like behavior and liver damage in mice. Pharmacol Biochem Behav 2015; 132: 79-87.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265-275.