| Bakuchiol, 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium-bromide (MTT) were procured from Sigma-Aldrich (St. Louis, MO, USA). Bakuchiol was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) to get a 100 mM stock solution, which was diluted in the medium to yield the anticipated concentration. An equivalent volume of DMSO in complete culture medium was used as the vehicle control. To exclude the cytotoxicity of DMSO, the ultimate concentration of DMSO for all experiments was kept at less than 0.2%. Minimum essential medium (MEM) and RPMI, fetal bovine serum (FBS), penicillin, streptomycin, trypsin, phosphate-buffered saline (PBS) with calcium chloride and magnesium chloride were obtained from Hangzhou Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou, China). Propidium iodide (PI), acridine orange (AO), Hoechst 33258 were purchased from Boster Biological Technology Co., Ltd. (Wuhan, China). |
| The SGC-7901 human gastric cancer cells were seeded on a 96-well plate at 2 × 105 cells per well. After 24 h, the cells were treated with bakuchiol at several doses (0, 10, 30, 50, and 100 µM). After incubation times of 12, 24 and 48 h, MTT solution (20 µl) was added. The formazan crystals thus formed were dissolved with DMSO and the absorbance was measured on a microplate reader (FLUOstar Optima, Offenburg, Germany). |
Phase contrast microscopy
|
| SGC-7901 human gastric cancer cells were plated in sixwell plates at a density of 2 × 105 cells/ml and then cultured for 24 h. Subsequently, the cells were exposed to treatment with various concentrations of bakuchiol (0, 10, 50 and 100 µM) for 48 h. Following drug treatment, culture plates were examined using an inverted light microscope (Nikon Corp., Tokyo, Japan) and images were captured. DMSO was used as a control. |
Fluorescence microscopic assay using acridine orange (AO) and propidium iodide and Hoechst 33258 staining dyes
|
| SGC-7901 human gastric cancer cells were seeded on a chamber slide (Thermo Scientific Nunc Lab Tek II) at cell density of 2 × 105 cells per chamber. The cells were treated with 0, 10, 50 and 100 µM bakuchiol for 48 h. Afterwards, 10 µg/ml of acridine orange (AO) and 10 µg/ml of propidium iodide (PI) were added to each chamber. It was then observed under fluorescence microscope (Olympus IX-70, Tokyo, Japan).Further, after treating cells with above mentioned doses, the cells were washed with PBS and fixed with 3.5 % formaldehyde for 20 min. After that the cells were again washed removing the fixing solution and then stained with Hoechst 33258. The cells were again washed before analysis under a fluorescence microscope (Olympus IX 81 Tokyo, Japan). |
Cell cycle analysis by flow cytometry
|
| The cell cycle analysis was carried out by flow cytometry (Becton-Dickinson FACS Calibur flow cytometry) equipped with CellQuest 3.3 software. After incubation with bakuchiol for 48 h, SGC-7901 human gastric cancer cells were harvested, fixed with 70% ice-cold ethanol for 24 h, treated with 20 µg/ml RNase A (Sigma-Aldrich, St. Louis, MO, USA), stained with 20 µg/ml propidium iodide (PI), and then analyzed by flow cytometer. |
Statistical analysis
|
| The results designate values from three independent experiments with the data expressed as the means ± SD. Differences between the control and treatment groups were examined using the Student's t-test with SPSS 17.0 software. A p-value <0.05 was considered statistically significant. |
Results and Discussion
|
Bakuchiol induces potent cytotoxic effects in SGC-7901 human gastric cancer cells
|
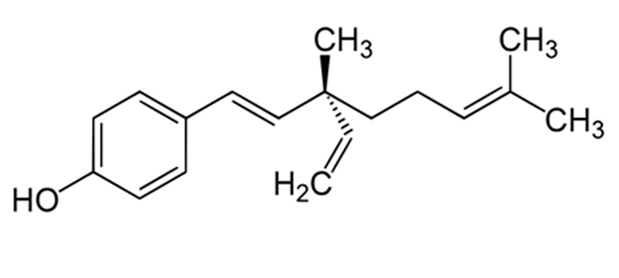 |
| Figure 1: Chemical structure of bakuchiol |
| The chemical structure and the cytotoxic effects of bakuchiol in SGC-7901 gastric cancer cells are shown in Figure 1 and Figure 2 respectively. As is evident from the figure, bakuchiol induced potent, concentration dependent as well as timedependent cytotoxic effects in SGC-7901 human gastric cancer cells. The IC50 values of bakuchiol at three different time intervals were found to be 58.4, 42.3 and 32.5 µM respectively at 12, 24 and 48 h time intervals respectively. This indicates that the cytotoxic effect of this compound increases with increase in the incubation time also. |
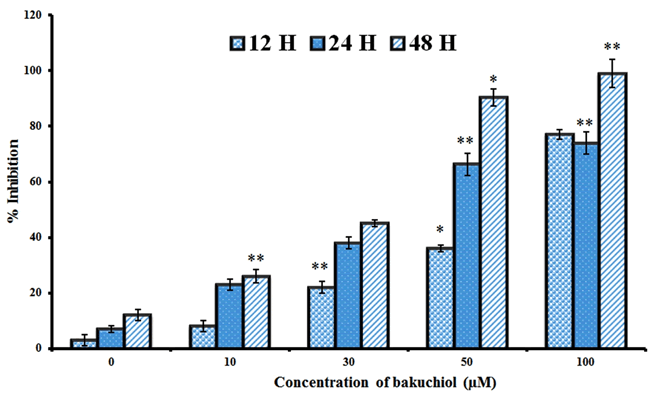 |
| Figure 2: Cytotoxic effect of bakuchiol on the proliferation of SGC-7901 gastric cancer cells. Data are shown as the mean ± SD of three independent experiments. *P<0.05, **P<0.01, vs 0 µM (control) |
Effect of bakuchiol on the cellular morphology of SGC-7901 human gastric cancer cells
|
| In this assay, SGC-7901 gastric cancer cells were exposed to increasing doses of bakuchiol in order to examine any morphological changes induced by this compound. The results of this assay are depicted in Figure 3 A-D indicating that untreated control cells exhibited normal morphology like round shape and were attached to one another. However, on treatment with 10, 50 and 100 µM dose of bakuchiol for 48 h, phase contrast microscope revealed that the cells got detached from one another making clusters of small number of cells floating in the medium. These cells also had uneven shape and incapable to maintain their intact membranes. |
 |
Bakuchiol induced characteristic morphological features of apoptosis
|
| Fluorescence microscopy using acridine orange and propidium iodide double staining indicated that bakuchiol induced morphological features which are the indications of apoptosis. The results of this assay are shown in Figure 4 A-D revealing that untreated SGC-7901 human gastric cancer cells showed green fluorescence. However, after the cells were treated with 10, 50 and 100 µM concentrations of bakuchiol, these cells began to emit orange red fluorescence more heavily at the centre of cells indicating apoptosis. The number of these apoptotic cells increased with increase in the dosage of bakuchiol. |
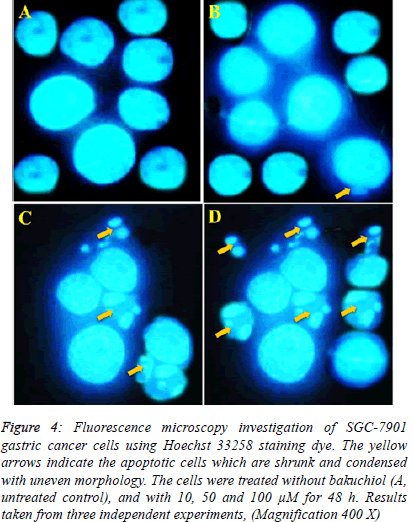 |
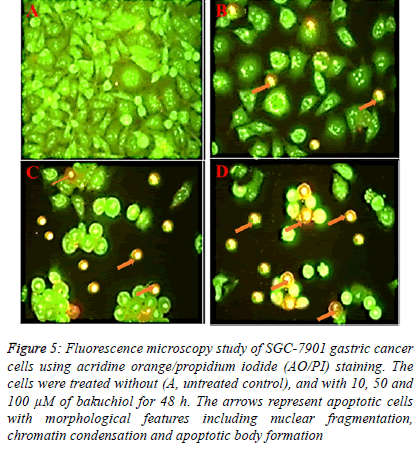
| In case of Hoechst 33258 staining, similar results indicating apoptosis were obtained. The results are shown in Figure 5 indicating that unlike control untreated cells which showed normal morphology and spherical shape (Figure 5 A). The bakuchiol-treated cells showed significant chromatin condensation, chromosomal DNA cleavage, blebbing of the membrane and formation of apoptotic bodies. The appearance of these apoptotic bodies was closely related to the dose of bakuchiol (Figure 5 B-D). |
 |
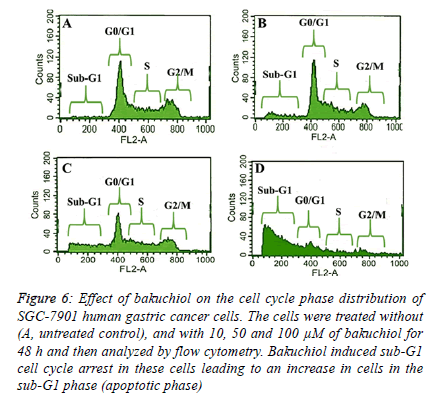
Bakuchiol induced sub-G1 cell cycle arrest in SGC-7901 cells
|
| The fact that bakuchiol induced apoptosis was further confirmed by flow cytometry using propidium iodide as a fluorescent probe. After the SGC-7901 cells were treated with 10, 50 and 100 µM dose of bakuchiol for 48 h, it was observed that there occurred an obvious accumulation of cells in the sub- G1 phase of the cell cycle (also called as the apoptotic phase). The results which are shown in Figure 6 A-D reveal that as compared to the untreated control cells which only showed 1.3% of cells in sub-G1 phase, the cells treated with 10, 50 and 100 µM dose of bakuchiol indicated that the percentage of cells in the sub-G1 phase increased significantly to 6.5%, 23.8% and 62.2% respectively. |
Discussion
|
| In the present study, it was observed that bakuchiol induced potent cytotoxic effects in SGC-7901 human gastric cancer cells in a dose and time-dependent manner. Further, using phase contrast and fluorescence microscopic techniques, it was observed that bakuchiol could induce various morphological features which are characteristic of apoptosis including DNA fragmentation, chromatin condensation, blebbing of the membrane and formation of apoptotic bodies. On treatment with 10, 50 and 100 µM dose of bakuchiol for 48 h, phase contrast microscope revealed that the cells got detached from one another making clusters of small number of cells floating in the medium. The cells when treated with 10, 50 and 100 µM concentrations of bakuchiol, these cells began to emit orange red fluorescence more heavily at the centre of cells indicating apoptosis. Flow cytometry revealed that bakuchiol also induced sub-G1 cell cycle arrest. The cells treated with 10, 50 and 100 µM dose of bakuchiol indicated that the percentage of cells in the sub-G1 phase increased significantly to 6.5%, 23.8% and 62.2% respectively. |
 |
| Natural products have always been used as anticancer agents from many decades. Natural products have played significant role in the design and development of more than 60% of the clinically used anticancer agents. Furthermore, there are numerous natural products or their analogs which are currently in preclinical and clinical stage. World Health organization (WHO) has estimated that about 75 to 80% of the world population rely on traditional medicines for their main health care [6-8]. Natural products which are highly operative and possess less side-effects are a promising substitute for chemotherapy with deadly side-effects. The use of noncytotoxic bioactive molecules have a great prospective for using against cancer because most of these natural products exhibit pleiotropic properties [9]. |
| Bakuchiol is a terpenoid compound mostly isolated from Psoralea corylifolia and Otholobium pubescens [10]. Previous studies have reported that bakuchiol exhibits anticancer, hepatoprotective, antihyperglycemic and antibacterial activities [11-13]. To the best of our knowledge, no such study has been published reporting anticancer activity of bakuchiol in SGC-7901 human gastric cancer cells. Therefore, we undertook this objective to study the effect of this compound on this cell line along with studying its mode of action by investigating its effect on cellular apoptosis and cell cycle phase distribution. |
Conflict of interest
|
| The authors declare that there is no conflict of interest to reveal with regard to this research work. |
| Bakuchiol inhibits cell proliferation and induces apoptosis and cell cycle arrest in SGC-7901 human gastric cancer cells |
|
|
References
Muñoz N, Franceschi S.Epidemiology of gastric cancer and perspectives for prevention.SaludPublicaMex1997; 39: 318-330.
- Inoue M, Tsugane S.Epidemiology of gastric cancer in Japan. Postgrad Med J 2005; 81: 419-424.
- Lin HH, Huang HP, Huang CC, Chen JH, Wang CJ.Hibiscus Polyphenol-Rich Extract Induces Apoptosis in Human Gastric Carcinoma Cells via p53 Phosphorylation and p38 MAPK/FasL Cascade Pathway. MolCarcinog2005; 43: 86-99.
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer1972; 26: 239-257.
- Searle J, Lawson TA, Abbott PJ, Harmon B, Kerr JF. An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J Pathol1975; 116: 129-138.
- Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer 2002; 2:143-148.
- Fulda S. Modulation of apoptosis by natural products for cancer therapy. PlantaMedica 2010; 76:1075-1079.
- Petronelli A, Pannitteri G, Testa U.Triterpenoids as new promising anticancer drugs. Anticancer Drugs 2009;20:880-92.
- Slichenmyer WJ, Von Hoff DD.New Natural Products in Cancer Chemotherapy. J ClinPharmacol 1990; 30: 770-788.
- Banerji A, Chintalwar GJ.Biosynthesis of bakuchiol, a meroterpene from Psoraleacorylifolia. Phytochemistry 1983; 22:1945-1947.
- Cho H, Jun JY, Song EK, Kang KH, Baek HY, Ko YS, Kim YC.Bakuchiol: A Hepatoprotective Compound of Psoraleacorylifolia on Tacrine-Induced Cytotoxicity in Hep G2 Cells. Planta Med 2001; 67:750-751.
- Krenisky JM, Luo J, Reed MJ, Carney JR.Isolation and antihyperglycemic activity of bakuchiol from Otholobiumpubescens (fabaceae), a Peruvian medicinal plant used for the treatment of diabetes. Biol Pharm Bull 1999; 22:1137-1140.
- Chen Z, Jin K, Gao L, Lou G, Jin Y, Yu Y, Lou Y. Anti-tumor effects of bakuchiol, an analogue of resveratrol, on human lung adenocarcinoma A549 cell line. Eur J Pharmacol 2010; 643:170-179.
|
-
|





