ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 4
Anti-proliferative and apoptosis-inducing effects of active constituents in Bufo melanostictus Schneider on human gastric cancer MGC803 cells
1Tumor surgery, Chinese medicine hospital of Wenling City, Zhejiang Province 317500, China
2Obstetrics and Gynecology Department, Chinese medicine hospital of Wenling City, Zhejiang Province 317500, China;
3Department of Pathology, Chinese medicine hospital of Wenling City, Zhejiang Province 317500, China.
- *Corresponding Author:
- Jiabao Lin
Tumor surgery, Chinese medicine hospital
of Wenling City, Zhejiang Province 317500
China E-Mail: linjbzj@163.com
Accepted date: August 11 2015
To extract, isolate and identify active constituents in Bufo melanostictus Schneider, and to study their anti-proliferative and apoptosis-inducing effects on human gastric cancer MGC803 cells. Chemical constituents were isolated and purified by repeated silica gel column chromatography, ODS reverse phase column chromatography, preparative liquid chromatography and recrystallization, and structurally elucidated based on physicochemical properties & spectral data of compounds and reference literatures. Anti-proliferative effects of different concentrations of Bufo melanostictus Schneider extracts on gastric cancer MGC803 cells were detected by MTT, and their morphological changing effects on these cells were observed by microscopy. Four compounds were isolated from the skin of Bufo melanostictus Schneider. The anti-proliferative effects of Bufo melanostictus Schneider extracts on gastric cancer MGC803 cells were enhanced with increasing concentration and action time. 72 h after action of test solution in the high-dose group, proliferation inhibition rate of MGC-803 cells reached 77.5%. Under the microscope, firmly adherent MGC-803 cells with intact membranes were observed in the control group; cells were bright and clear, had good refraction, and were fully spread; uniform cytoplasmic distribution was observed as well. In various treatment groups, cell density became gradually sparse with increasing drug concentration, and cell surfaces were wrinkled. In the high-dose group, most cells were disrupted; cell morphology was not intact; cell refraction decreased; most cells were detached; chromatins were condensed, compacted and split into blocks; and the number of adherent cells decreased. Active constituents in Bufo melanostictus Schneider can induce apoptosis of gastric cancer MGC803 cells.
Keywords
Bufo melanostictus Schneider; cinobufagin; MGC803
Venenum bufonis can be sourced from two types of animals: Bufo bufo gargarizans Cantor and Bufo melanostictus Schneider. Bufo melanostictus Schneider contains a variety of chemical constituents, which have been proven by modern pharmacological studies to have cardiotonic, hypertensive, analgesic and antitumor pharmacological activities [1-4]. With the deepening of research, Bufo melanostictus Schneider extract has been found to exert anticancer effects by induction of tumor cell apoptosis and differentiation by inhibiting endothelial proliferation and angiogenesis, inhibition of inflammatory cytokine production and other ways [5-8].
With the overall revitalization of Chinese medicine, cancer treatment by Chinese medicine has gained rapid development. Especially since the 1990s, with the continuous advances in technology and the improving levels of modern isolation and purification technologies and pharmacological and toxicological research, dozens kinds of Chinese medical preparations have been widely applied in clinical practice. Particularly in cancer treatment, Chinese drugs have prominent effects in improving quality of life of patients and other aspects. Extraction or semi-synthesis of active constituents from Chinese drugs and research of antitumor mechanism of Chinese drugs using modern high-tech means have become hot research topics in the field of Chinese medicinal tumor treatment in China. In this paper, spectral characteristics of major active constituents in Bufo melanostictus Schneider are studied in depth, and their inhibitory effects on human gastric cancer cells are explored by MTT assay and observation of morphological changes.
Materials
Instruments and reagents
Bruker AV 400 MHz superconducting NMR spectrometer; Varian prostar preparative HPLC system; SANYO MCO-175 CO2 incubator, Japan; NIKON ECLIPSE TE2000-U inverted microscope, Japan; BioTEK ELX 800 microplate reader, USA; BD FACSAria flow cytometry, USA. FBS, HYCLONE; RPMI 1640 medium, GIBCO; MTT, SIGMA.
Drug and cell lines
Bufo melanostictus Schneider was purchased from Anguo Market, which was identified as Bufo melanostictus Schneider. Human gastric cancer MGC803 cells were purchased from Wenling Hospital of Traditional Chinese Medicine in Zhejiang.
Methods
Extraction and isolation
5 kg of Bufo melanostictus Schneider skin was crushed, and extracted for 1 h with an 8-fold amount of water twice. Extracts were combined, concentrated, and precipitated twice with 80% ethanol, then, ethanol was removed to give the extract. Next, the extract solution was extracted successively with chloroform and ethyl acetate to give respective extracts. Afterwards, the chloroform and ethyl acetate extracts were loaded on silica gel column for isolation by preparative liquid chromatography, preparative thin layer chromatography and repeated Sephadex LH-20 column chromatography to obtain four compounds. Among them, compounds 1 and 2 were from chloroform fraction, whereas compounds 3 and 4 were from ethyl acetate fraction.
Cell culturing
Gastric cancer MGC803 cells were seeded in a culture flask with RPMI 1640 medium containing 10% FBS, and passaged under 5% CO2, 37°C conditions. Cells in the logarithmic phase were collected for experiments.
Preparation of test solutions
The above chloroform extracts were prepared into different concentrations of Bufo melanostictus Schneider test solutions (BSL, with cinobufagin contents of 0.05, 0.1 and 0.5 mg/ml, respectively) for assay of MGC803 cell viability.
MTT assay
Logarithmic phase MGC803 cells were digested with 0.25% trypsin, prepared into a 5×104 cells/mL cell suspension, inoculated in a 96-well plate at 1.0×104 cells per well, and treated with different concentrations of BSL (cinobufagin contents of 0.05, 0.1 and 0.5 mg/ml, respectively). Five parallel wells were set up for each concentration. At 72 h, 20 μl of MTT solution was added to each well, and incubation was continued for an additional 4 h. Then, supernatant was discarded, and each well was added with 150 μL of DMSO and shaken for 10 min. Finally, absorbance was measured at 570 nm using microplate reader, and cell growth inhibition rate was calculated.
Inhibition rate = (A value of negative control group - A value of treatment group) / A value of negative control group × 100%.
Observation of cell morphological changes by inverted microscope Logarithmic phase MGC803 cells were seeded in a 6-well plate at a 5×104/ml density to make cell slides. After adherence, MGC803 cells were treated with 0.5 mg/ml cinobufagin-containing BSL, and cultured under 5% CO2, 37°C conditions, followed by observation under an inverted microscope.
Statistical analysis
Data were processed using SPSS 13.0 statistical software. Measurement data were expressed as x — ±s. Comparison among groups was performed by t test, and P<0.05 was considered statistically significant.
Results
Structural elucidation of active constituents
Compound 1
White square crystals (acetone), mp 259-260℃, freely soluble in chloroform and methanol. ESI-MS (m/z): 443[M+H]+。 1H-NMR (CDCl3, J=Hz) δ(ppm): 7.95 (1H, d, J=10.0 Hz, H-22), 7.21 (1H, s, H-21), 6.18 (1H, d, J=10.0 Hz, H-23), 5. 52 (1H, d, J=9.5 Hz, H-16), 4.18 (1H, brs, H-3), 3.62 (1H, s, H-15), 2.76 (1H, d, J=9.4 Hz, H-17), 1.93 (3H, s, Ac), 1.07 (3H, s, H-19), 0.94 (3H, s, H-18); 13C-NMR (75 MHz, DMSO-d6) δ: 30.2 (C-1), 28.6 (C-2), 67.4 (C-3), 34.2 (C-4), 37.2 (C-5), 27.5 (C-6), 21.2 (C-7), 34.5 (C-8), 41.1 (C-9), 36.8 (C-10), 21.4 (C- 11), 40.2 (C-12), 46.9 (C-13), 73.2 (C-14), 63.4 (C-15), 73.5 (C-16), 53.6 (C-17), 17.9 (C-18), 24.8 (C-19), 120.7 (C-20), 152.9 (C-21), 151.4 (C-22), 113.7 (C-23), 164.8 (C-24). The above data were consistent with the cinobufagin reported in the literature [9], so compound 1 was identified as cinobufagin.
Compound 2
White needle crystals (methanol), mp 218-221°C, freely soluble in chloroform. ESI-MS (m/z): 403[M+H]+. 1HNMR (CD3OD) δ(ppm): 7.76 (1H, dd, J=9.5, 2.4 Hz, H- 22), 7.31 (1H, d, J=2.5Hz, H-21), 6.27 (1H, d, J=9.5 Hz, H-23), 4.48 (1H, t, J=6.6 Hz, H-16), 4.16 (1H, brs, H-3), 2.67 (1H, d, J=7.5Hz, H-15), 2.46 (1H, d, J=6.6Hz, H-16), 2.41 (1H, d, J=6.4 Hz, H-17), 0.94 (3H, s, H-19), 0.85 (3H, s, H-18); 13C-NMR (75 MHz, DMSO-d6) δ: 33.6 (C- 1), 30.1 (C-2), 67.5 (C-3), 34.5 (C-4), 39.5 (C-5), 28.6 (C- 6), 22.4 (C-7), 42.9 (C-8), 42.7 (C-9), 37.4 (C-10), 69.5 (C-11), 51.2 (C-12), 50.7 (C-13), 84.9 (C-14), 33.8 (C- 15), 29.6 (C-16), 52.6 (C-17), 18.5 (C-18), 24.9 (C-19), 125.2 (C-20), 150.4 (C-21), 150.4 (C-22), 115.4 (C-23), 164.8 (C-24). The above data were consistent with the desacetylbufotalin reported in the literature [10], so compound 2 was identified as desacetylbufotalin.
Compound 3
White powder (methanol), mp 213-215°C, freely soluble in methanol. EIS-MS (m/z): 403[M+H]+. 1H-NMR (CD3OD) δ(ppm): 7.97 (1H, dd, J=9.5, 2.4 Hz, H-22), 7.48 (1H, d, J=2.6Hz, H-21), 6.24 (1H, d, J=9.6 Hz, H- 23), 4.11 (1H, brs, H-3), 3.62 (1H, dt, J=5.5 Hz, H-11), 2.35 (1H, dd, J=10.8, 5.5Hz, H-17), 1.12 (3H, s, H-19), 0.78 (3H, s, H-18); 13C-NMR (75MHz, DMSO-d6) δ: 30.5 (C-1), 28.6 (C-2), 67.4 (C-3), 41.2 (C-4), 36.2 (C-5), 27.5 (C-6), 22.3 (C-7), 40.1 (C-8), 37.8 (C-9), 36.2 (C-10), 21.4 (C-11), 40.5 (C-12), 46.9 (C-13), 73.2 (C-14), 73.1 (C-15), 63.5 (C-16), 53.6 (C-17), 17.8 (C-18), 24.6 (C- 19), 119.5 (C-20), 152.6 (C-21), 151.2 (C-22), 113.7 (C- 23), 164.9 (C-24). The above data were consistent with the 11β-hydroxyl-bufalin reported in the literature [9], so compound 3 was identified as 11β-hydroxyl-bufalin.
Compound 4
White solid (methanol), mp 194-196°C, freely soluble in chloroform. ESI-MS (m/z): 581 [M+ Na]+. 1H-NMR (CDCl3) δ(ppm): 7.85 (1H, dd, J=9.5, 2.7 Hz, H-22), 7.26 (1H, d, J=2.5Hz, H-21), 6.24 (1H, d, J=9.8Hz, H-23), 5.24 (1H, brs, H-3), 2.43 (1H, dd, J=9.5, 6.5 Hz, H-17), 1.07 (3H, s, H-19), 0.78 (3H, s, H-18); 13C-NMR (75 MHz, DMSO-d6) δ: 162.6 (C-24), 148.8 (C-21), 146.2 (C-22), 122.7 (C-20), 115.8 (C-23), 85.6 (C-14), 73.9 (C-5), 71.5 (C-3), 51.3 (C-17), 48.7 (C-13), 41.9 (C-8), 40.2 (C-12), 40.1 (C-10), 39.4 (C-9), 35.7 (C-4), 34.2 (C-6), 32.4 (C- 15), 28.3 (C-16), 28.8 (C-2), 25.8 (C-1), 23.5 (C-7), 21.4 (C-11), 16.8 (C-19), 16.6 (C-19), 172.5 (C-1′), 33.3 (C- 2′), 24.7 (C-3′), 24.8 (C-4′), 24.9 (C-5′), 29.8 (C-6′), 34.6 (C-7′), 177.5 (C-8′). The above data were consistent with the telocinobufagin-3-monosuberate reported in the literature [11], so compound 4 was identified as telocinobufagin-3-monosuberate.
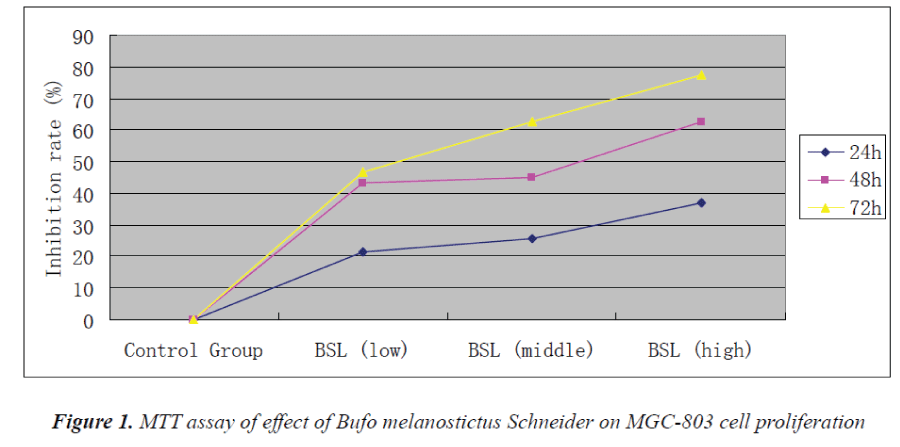
MTT assay results
Experimental results showed that Bufo melanostictus Schneider could evidently inhibit the proliferation of MGC-803 cells. Its anti-proliferative effect on gastric carcinoma MGC803 cells was enhanced with increasing concentration and action time. 72 h after action of test solution in the high-dose group, proliferation inhibition rate of MGC-803 cells reached 77.5%.
Effect of Bufo melanostictus Schneider extracts on MGC-803 cell morphology
Under the microscope, firmly adherent MGC-803 cells with intact membranes were observed in the control group; cells were bright and clear, had good refraction, and were fully spread; uniform cytoplasmic distribution was observed as well. In various treatment groups, cell density became gradually sparse with increasing drug concentration, and cell surfaces were wrinkled. In the high-dose group, most cells were disrupted; cell morphology was not intact; cell refraction decreased; most cells were detached; chromatins were condensed, compacted and split into blocks; and the number of adherent cells decreased.
Discussion
Cancer has become an intractable illness seriously threatening human life and health, whose hazard to human health ranks second only to cardiovascular diseases. Under adverse external environments and a number of unfavorable factors, the incidence of cancer has been gradually increasing in the clinical settings along with continuous increase of life habits and stress. Vigorous development of modern medicine offers great possibilities for effective control and cure of cancer. In recent years, outcomes of tumor therapies such as surgical resection and radiotherapy have not been very satisfactory, and recurrence and metastasis of cancer cells have brought people's attention to traditional drug therapies. Therefore, the search for low-toxic and efficient natural anticancer drugs has been the key and focus of research in recent years.
Apoptosis, also known as programmed cell death, is an autonomous cell death process controlled by genes, which is one of the important mechanisms for maintaining homeostasis. Apoptosis is an orderly, controlled physiological process of natural cell death that develops in accordance with a specific program. Its morphological characteristics are as follows: at first, cytoplasm becomes rounded, and then detaches from the adjacent cells, loses microvilli, and condenses; endoplasmic reticulum dilates, bubbles, and fuses with the cell membranes; mitochondria have no major changes; nuclear chromatins compact, form crescent shapes, and condense around the nuclear membranes; nucleoli are cleaved, and thereby leads to membrane invagination, spontaneously decomposing cells into multiple membrane-packed apoptotic bodies. As a complex process, apoptosis is an initiative cell suicidal process during growth, differentiation, developmental and pathological processes of organisms. Organisms clear those senescent, aberrant and potential disease-causing cells through apoptosis, and thereby maintain the normal organism operation.
A significant sign during apoptosis is the ordered DNA breakage, which is also an important distinction from necrosis. After treatment of human gastric cancer MGC- 803 cells with different concentrations of Bufo melanostictus Schneider extracts, cell shrinkage and apoptotic bodies are observed under the microscope. Cells in the treatment groups have partial chromosomal condensation and membrane blebbing, wrinkled cell surfaces and disintegrated cell morphology. Meanwhile, fragment nuclei are visible. Compared with the dense nuclei of normal cells, it can be speculated that these cells have undergone apoptotic cell death.
References
- Kelly B, Paul A, Tousif P, Kathleen E, Jan P, Keith DL.Incidence and risk factors for cholangiocarcinoma in primary selemsing cholangitis. Am J Gastmenterol2004; 99: 523-526.
- Lazaridis KN, Gores GJ. Cholangiocarcinoma.Gastroenterology 2005; 128: 1655-1667.
- Chen XY, Xu RC, Chen L, Qian J. Studies on the Cytotoxicity of Bufalin on Human Gastric Cancer Cell MGc-803 in Vitro. Chinese Traditional and Herbal Drugs 2000; 31: 920-922.
- Takai N, Ueda T, Nishida M, Nasu K, Narahar H. Bufalin induces growth inhibition, cell cycle arrest and apoptosis in human endometrial and ovarian cancer cells. Int J Mol Med 2008; 26: 186-188.
- Han KQ, Huang G, Gu W, Su YH, Huang XQ, Ling CQ. Anti-tumor activities and apoptosis-regulated mechanisms of bufalin on the orthotopic transplantation tumor model of human hepatocellular carcinoma in nude mice. World J Gastroenterol 2007; 13: 3374-3379.
- Kurosawa M, Numazawa S, Tani Y, Yoshida T. ERK signaling mediates theinduction of inflammatory cytokines by bufalin in human monocytic cells. Am J Physiol Cell Physiol 2000; 278: C500-C508.
- Numazawa S, Inoue N, Nakura H, Sugiyama T, FujinoE, Shinoki M, Yoshida T, Kuroiwa Y. A cardiotonicsteroid bufalin-induced differentiation of THP-1 cells.Involvement of Na+, K+-ATPase inhibition in the early changes in proto-oncogene expression.BiochemPharmacol 1996; 52: 321-329.
- Bhuiyan MB, Fant ME, Dasgupta A. Study on mechanism of action of Chinese medicine Chan Su:dose-dependent biphasic production of nitric oxide in trophoblastic BeWo cells. ClinChimActa 2003; 330: 179-184.
- Ye M, Qu GQ, GuoHZ,Guo DA. Specific 12β- Hydroxylation of cinobufagin by Filamentous Fungi. App l Environ Microbiol 2004; 70: 3521-3527.
- Zhan JX, Liu WH, Guo HZ, Zhang YX, Guo DA.Selective dehydrogenation of resibufogenin and cinobufagin at 3-OH by P seudomonasaeruginosa.Enzyme MicrobTechnol 2003; 33: 29-32.
- Shimada K, Nambara T. Isolation and characterization of a new type of bufotoxin from the skin of Bufo americanus. Chem Pharm Bull 1980; 28: 1559-1562.