ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 4
Anti-proliferative activity of active constituents in Veratrum dahuricum on human bladder cancer T24 cells
1Department of Surgery II, Dongzhimen Hospital Beijing University of Chinese Medicine, Beijing China;
2Zhanlanlu Community Health Service Centers in Xicheng District Beijing, Beijing China.
#Equal contributors to this work
- *Corresponding Author:
- Yanfeng Li
Department of Surgery II
Dongzhimen Hospital of Beijing University of Chinese
Medicine Haiyuncang 5 of Beijing Dongcheng District
Beijing, China.
Accepted June 14 2014
To analyze the main chemical constituents of Veratrum dahuricum, and study their antibladder cancer activity. Chloroform and ethyl acetate fractions are obtained by ethanol extraction, and subsequent extraction with chloroform, ethyl acetate, saturated water and n-butanol. The fractions are then further purified through silica gel column chromatography, preparative HPLC, repeated silica gel column chromatography (chloroform-methanol), D101 macroporous resin column chromatography, Sephadex LH-20 gel column chromatography (methanol), C-18 reversed-phase silica gel column chromatography (methanol-water), preparative TLC and recrystallization. Inhibitory effect of resveratrol on T24 cells is observed by MTT assay and inverted microscopy. Six compounds are isolated from Veratrum dahuricum, namely verzine, germitrine, quercetin, stigmasterol, polydatin and resveratrol. MTT assay results show that the test concentrations of resveratrol can effectively inhibit the proliferation of T24 cells. The inhibitory effect is enhanced gradually with increasing drug concentration, showing a certain dose-response relationship. Under inverted microscope, adherently growing cells are visible in the control group, with tight and full intercellular junction and intact membranes. In the test group, cell growth density becomes gradually sparse with increasing drug concentration, intercellular junction is loose, adherent capacity is clearly insufficient, cell surface is wrinkled, and cell debris is also plentiful. In the high concentration group, most cells are disrupted, cell morphology is incomplete, and adherent cells are reduced in number. Veratrum dahuricum can inhibit the proliferation of human bladder cancer T24 cells, which has a certain anti-cancer activity.
Keywords
Veratrum dahuricum, Resveratrol, T24 cell
Introduction
Veratrum nigrum L., also known as Heililu or Shancong, is a perennial herb in the genus Veratrum of the family Liliaceae. It is mainly grown in valleys or hillsides, and distributed in China's northeastern region, Hebei, Shandong, Henan and other places. The herb is used in the treatment of hypertension, schistosomiasis, hookworm, filariasis, stroke, phlegm obstruction, throat impediment, epilepsy, malaria, fractures, scabies, malignant sores, etc. Main constituents of Veratrum nigrum L. are steroidal alkaloids, stilbenes and flavonoids, which include jervines, cevines, solasodines, veratrines, resveratrol, quercetin, isorhamnetin, 4'-methoxy isorhamnetin, isoquercitrin, etc [1-2].
Modern pharmacological studies have confirmed that Veratrum plants have rather remarkable anti-tumor activity [3-5], which is achieved by inhibition of Hedgehog signaling pathway transduction and expression via Smo antagonism by cyclopamine, thereby leading to inhibition of cancer cell proliferation. In addition, Veratrum plants also have blood pressure lowering, hemorheology influencing and anti-fungal effects [6-8].
This paper extracts, isolates, purifies and structurally elucidates the chemical constituents of Veratrum dahuricum, and studies the anti-cancer activity of resveratrol isolated, with the aim of providing scientific support for effective clinical treatment of bladder tumors.
Material
Instruments and reagents
NMR spectrometer (Bruker DRX-500); melting point detector (model RY-2, uncorrected, Tianjin Analytical Instrument Factory); mass spectrometer: Mat-212 magnetic (El-MS); Q-Tof micro (ESl-MS). Reagents used were of analytical grade. RPMI 1640 (Gibco); FBS (Hangzhou Sijiqing Biologicals); MTT (Sigma).
Drug and cells
Veratrum dahuricum was purchased from the medicine market in Anguo; and resveratrol, with a purity above 98%, was self prepared. Human bladder transitional cell carcinoma T24 cell line was purchased from Shanghai Institutes for Biological Sciences, CAS.
Methods
Extraction and isolation
10 kg of Veratrum dahuricum was pulverized into a coarse powder, and reflux extracted with an 8-fold amount of 80% ethanol for 2 h three times. Then, ethanol was removed, and the extract was dissolved with 5% aqueous tartaric acid, extracted with diethyl ether, and pH adjusted to 9 with 10% NaOH solution to obtain the precipitate and lye parts. Next, the precipitate part was extracted successively with chloroform, ethyl acetate, saturated water and n-butanol to give respective extracts.
The chloroform fraction was loaded on silica gel column, and gradient eluted with chloroform-methanol. Then, similar fractions were combined, and further purified by preparative HPLC to give compounds 1-3.
Ethyl acetate fraction was subjected to repeated silica gel column chromatography (chloroform-methanol), D101 macroporous resin column chromatography, Sephadex LH-20 gel column chromatography (methanol), C-18 reversed- phase silica gel column chromatography (methanol- water ), preparative TLC and recrystallization to give compounds 4-6.
Cell culturing
Human bladder transitional cell carcinoma T24 cell lines were cultured in 10% FBS-containing RPMI 1640 medium, and passaged routinely in a 37℃, 5% CO2 incubator. Logarithmic phase cells were used for the experiment.
Determination of the effect of resveratrol on T24 cell proliferation by MTT assay
Logarithmic phase T24 cells (concentration of 1×104/mL) were seeded in 96-well plates at 100 μL per well, cultured in a 37℃, 5% CO2 incubator for 24 h, then added with different concentrations of resveratrol test solutions (50 umol/L, 100 umol/L and 200 umol/L). Six parallel wells were set up for each concentration, and control group was added with an equivalent volume of culture medium. After culturing for another 48 h, supernatant was discarded, 100 μL of 0.5 mg/mL MTT solution was added, and the culturing was continued for an additional 4 h. Finally, A value was measured with a microplate reader, and cell inhibition rate was calculated.
Inhibition rate = (A value of control group - A value of treatment group) / A value of control group
Observation of cell morphological changes under inverted microscope
Logarithmic phase T24 cells were concentration adjusted to 5×105/mL, cultured for 24 h, and then added with resveratrol test solution (100 umol/L). After culturing in a 37℃, 5% CO2 incubator for 24 h, cell morphology was observed under an inverted microscope (10×40 magnification).
Statistical methods
Analysis was done using SPSS13.0 statistical software; comparison among groups was performed by ANOVA, and pairwise comparison was done by t test. P<0.05 was considered statistically different.
Results
Structure elucidation of compounds
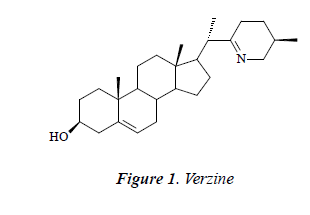
Compound 1: white powder, orange by Dragendorff reagent, and positive to bismuth potassium iodide reaction, indicating that the compound may be an alkaloid. 1H-NMR (CD3OD): δ 6.78 (1H, brs), 6.35 (1H, t, J = 1.6, 2.5 Hz), 6.65 (1H, brs), 7.36 (2H, d, J = 8.0 Hz), 6.81 (2H, t, J = 8.5 Hz), 6.80 (1H, d, J = 15.8 Hz), 7.14 (2H, d, J= 8.5 Hz), 4.80 (1H, d, J = 7.5 Hz), 3.79 (1H, d, J = 12.5 Hz), 3.48 (1H, d, J = 12.5 Hz), 3.36 (1H, dd, J =10.5, 2.4 Hz), 3.19-3.35 (2H, m), 3.21 (1H, td, J = 4.4, 3.8, 4.8 Hz); 13C-NMR (DMSO-d6) δ: 37.5 (C-1)s, 32.6 (C-2), 71.9 (C-3), 41.8 (C-4), 142.5 (C-5), 121.9 (C-6), 33.3 (C-7), 32.5 (C-8), 50.9 (C-9), 37.8 (C-10), 23.1 (C- 11), 39.3 (C-12), 43.7 (C-13), 57.1 (C-14), 27.1 (C-15), 26.1 (C-16), 53.9 (C-17), 20.2 (C-18), 12.9 (C-19), 47.8 (C-20), 19.3 (C-21), 175.2 (C-22), 26.0 (C-23), 27.8 (C- 24), 56.9 (C-25), 57.6 (C-26), 18.6 (C-27). After comparison with the literature [9], the structure of compound 1 was identified as verzine.
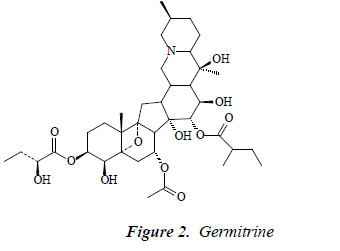
Compound 2: white powder, orange by Dragendorff reagent, and positive to bismuth potassium iodide reaction, indicating that the compound was an alkaloid. 1H-NMR (CD3OD): δ 6.78 (1H, brs), 6.39 (1H, t, J = 1.8, 2.5 Hz), 6.61 (1H, brs), 7.49 (2H, d, J = 8.5Hz), 6.89 (2H, t, J = 8.0 Hz), 6.87 (1H, d, J = 16.5 Hz), 7.12 (2H, d, J = 8.5 Hz), 4.81 (1H, d, J = 7.5 Hz), 3.69 (1H, d, J = 12.5 Hz), 3.63 (1H, d, J = 12.5 Hz, 3.39 (1H, dd, J =9.5, 2.6 Hz), 3.23-3.40 (2H, m), 3.22 (1H, td, J = 4.5, 3.8, 5.0 Hz); 13C-NMR (75 MHz, DMSO-d) δ: 32.8 (C- 1), 27.4 (C-2), 76.1 (C-3), 105.1 (C-4), 47.3 (C-5), 29.9 (C- 6), 67.2 (C-7), 47.2 (C-8), 95.7 (C-9), 45.9 (C-10), 34.2 (C- 11), 46.9 (C-12), 33.9 (C-13), 81.8 (C-14), 68.8 (C-15), 71.1 (C-16), 45.4 (C-17), 62.1 (C-18), 19.7 (C-19), 73.2 (C-20), 20.8 (C-21), 71.2 (C-22), 19.3 (C-23), 29.6 (C-24), 28.6 (C- 25), 61.3 (C-26), 17.4 (C-27), 3-OMB: 175.8 (C-1’), 42.1 (C-2’), 17.5 (C-3’), 26.3 (C-4’), 11.1 (C-5’), 15-OHMB: 176.5 (C-1’’), 75.0 (C-2’’), 25.8 (C-3’’), 34.3 (C-4’’), 7.9 (C- 5’’). After comparison with the literature [10], the structure of compound 2 was found basically consistent with the known compound germitrine, so compound 2 was germitrine.
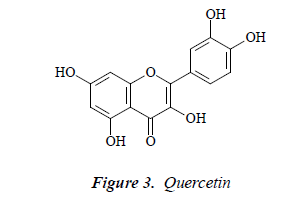
Compound 3: yellow needle crystals. Molecular ion peak by ESI-MS was m/z 303. 1H-NMR (DMSO-d6): δ 12.55 (1H, s, OH-5), 7.59 (1H, d, J=2.5 Hz, H-2'), 7.52 (1H, dd, J=8.5, 2.5 Hz, H-6'), 6.98 (1H, d, J=8.5 Hz, H-5’), 6.51 (1H, d, J=2.5 Hz, H-8), 6.31 (1H, d, J=2.5 Hz, H-6); 13CNMR (75 MHz, DMSO-d6) δ: 145.5 (C-2), 136.2 (C-3), 175.7 (C-4), 156.9 (C-5), 98.9 (C-6), 164.5 (C-7), 93.6 (C-8), 161.4 (C-9), 102.9 (C-10), 121.4 (C-1’), 116.2 (C- 2’), 146.5 (C-3’), 148.1 (C-4’), 115.2 (C-5’), 120.9 (C-6’). After comparison with the literature [11], the structure of compound 3 was found basically consistent with quercetin, so compound 3 was quercetin.
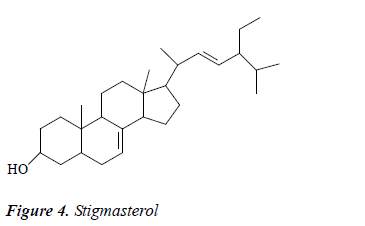
Compound 4: colorless needle crystals, positive to Liebermann-Burchard reaction. 1H-NMR (CDCl3): δ 5.23 (2H, m, H-7, 22), 5.12 (1H, dd, J = 15Hz, 8.5 Hz, H-23), 1.11, 0.89, 0.83 (3H, d, CH3×3), 0.85 (3H, t, CH3), 0.83, 0.58 (3H, s, CH3×2); 13C-NMR (CDCl3): δ 140.1 (C-8), 139.2 (C-21), 128.5 (C-22), 119.1 (C-7), 71.2 (C-3), 56.8 (C-17), 55.9 (C-14), 52.6 (C-24), 44.1 (C-13), 43.6 (C-9), 40.2 (C-20), 41.2 (C-5), 39.9 (C-12), 38.7 (C-4), 37.9 (C- 1), 34.5 (C-10), 31.2 (C-26), 32.2 (C-2), 29.1 (C-6), 28.5 (C-16), 24.9 (C-28), 22.8 (C-15), 22.2 (C-11), 20.9 (C- 27), 21.7 (C-25), 19.6 (C-21), 14.9 (C-19), 11.9 (C-18), 12.6 (C-29). After comparison with the literature [12], compound 4 was identified as stigmasterol.
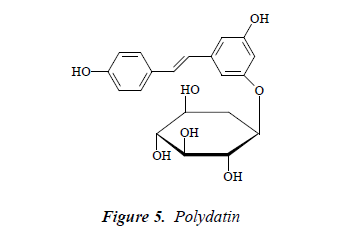
Compound 5: white needle crystals, positive to Molish reaction. ESI-MS m/z 413. 1H-NMR (CD3OD): δ 6.74 (1H, brs), 6.35 (1H, t, J = 1.8, 2.4 Hz), 6.58 (1H, brs), 7.43 (2H, d, J = 8.0 Hz), 6.86 (2H, t, J = 8.0 Hz), 6.88 (1H, d, J = 16.0 Hz), 7.11 (2H, d, J= 8.0 Hz), 4.83 (1H, d, J = 7.0 Hz), 3.71 (1H, d, J = 12.2 Hz), 3.51 (1H, d, J = 12.0 Hz), 3.33 (1H, dd, J = 9.6, 2.4 Hz), 3.22-3.37 (2H, m), 3.20 (1H, td, J = 4.2, 3.6, 4.8 Hz); 13C-NMR (75 MHz, DMSO-d6): δ 139.7 (C-1), 105.1 (C-2), 159.1 (C- 3), 101.7 (C-4), 159.2 (C-5), 106.8 (C-1’), 127.7 (C-2’), 116.5 (C-3’), 115.6 (C-4’), 116.1 (C-5’), 127.2 (C-6’), 101.0 (C-1’’), 73.8 (C-2’’), 77.5 (C-3’’), 68.8 (C-4’’), 76.4 (C-5’’), 61.5(C-6’’). After comparison with the literature [13], and combined with 1H NMR and 13C-NMR spectral data, compound 5 was identified as polydatin.
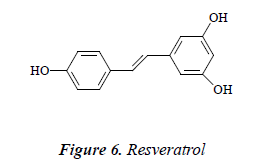
Compound 6: pale yellow needle crystals, quasimolecular ion peak by ESI-MS was m/z 229 [M+H]. 1H NMR (CD3OD): δ 6.88 ( H, d, J = 16.5 Hz), 7.03 (1H, d, J = 16 Hz), 6.54 (2H, d, J = 2.0 Hz), 6.27 (1H, t, J = 2.0 Hz), 6.87 (2H, d, J = 8.0 Hz), 7.45 (2H, d, J = 8.0 Hz); 13C-NMR (75 MHz, DMSO-d6) δ: 140.9 (C-1), 105.4 (C- 2), 159.4 (C-3), 102.1 (C-4), 158.7 (C-5), 106.2 (C-6), 130.2 (C-1’), 128.7 (C-2’), 116.6 (C-3’), 157.1 (C-4’), 117.1 (C-5’), 128.9 (C-6’). The above data were basically consistent with the known compound resveratrol [14], so the structure of compound 6 was identified as resveratrol.
MTT assay results
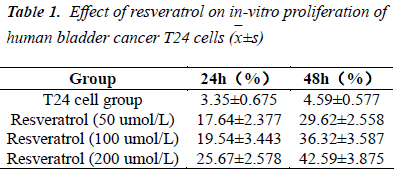
Determination of structure by MTT assay demonstrated that the test concentrations of resveratrol could effectively inhibit the proliferation of human bladder cancer T24 cells. Moreover, the inhibitory activity was enhanced with increasing concentration of test solutions; and 48 h after treatment with 200 umol/L test solution, the inhibition rate reached 42.59%. These indicated that resveratrol could significantly inhibit T24 cell proliferation, whose inhibitory effect was time- and dose-dependent.
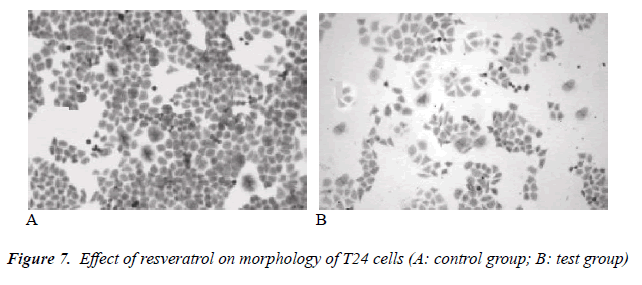
Effect of resveratrol on morphology of human bladder cancer T24 cells
Under inverted microscope, adherently growing cells were visible in the control group, with tight and full intercellular junction and intact membranes. In the test group, cell growth density became gradually sparse with increasing drug concentration, intercellular junction was loose, adherent capacity was clearly insufficient, cell surface was wrinkled, and cell debris was also plentiful. In the high concentration group, most cells were disrupted, cell morphology was incomplete, and adherent cells were reduced in number. The results are shown in Figure 7.
Discussion
Veratrum dahuricum contains rather many constituents, which are mainly steroidal alkaloids, flavonoids and stilbenes. But due to the low content of each compound, isolation and purification are rather difficult. During isolation, the flavonoidal compounds (quercetin, etc.) obtained have quite stable structures, so they are less demanding on the experimental conditions. But for cevine-based steroidal alkaloids, most contain 3, 7 and 15 acetyl and isovaleryl groups, and veratroyl and angeloyl groups are replaced, so their structures are instable. During isolation and purification, monomer components are highly prone to deacetylation reaction in solution, so the instability of these components is evident, which greatly affects the isolation, purification and structure elucidation. By rapid elution, low-temperature drying, crystallization and other methods, supplemented by modern means such as NMR, isolation is completed smoothly to ultimately obtain six compounds.
Bladder cancer is the most common cancer of the urinary system in China, which accounts for 3.2% of all malignancies. Transitional cell tumors make up over 90% of all bladder cancers, which seriously threat the survival of patients. Recent epidemiological survey shows that bladder cancer is on the rise, so the search for appropriate drugs, especially TCM preparations to alleviate patients' pain has become an important issue faced by researchers.
In this experiment, the effect of resveratrol on apoptosis of human bladder cancer T24 cells is observed. MTT assay results show that the test concentrations of resveratrol can effectively inhibit the proliferation of T24 cells. The inhibitory effect is enhanced gradually with increasing drug concentration, showing a certain dose-response relationship. Under inverted microscope, adherently growing cells are visible in the control group, with tight and full intercellular junction and intact membranes. In the test group, cell growth density becomes gradually sparse with increasing drug concentration, intercellular junction is loose, adherent capacity is clearly insufficient, cell surface is wrinkled, and cell debris is also plentiful. In the high concentration group, most cells are disrupted, cell morphology is incomplete, and adherent cells are reduced in number. The apoptosis-inducing mechanism of resveratrol on T24 cells needs further confirmation.
References
- Zhao WJ, Meng QW, Wang SS. Study on the alkaloidalconstituents of VeratrumschindleriLoes. China Journal
- of Chinese MateriaMedica 2003; 28: 985-986.
- Zhou CX, Liu JL, Ye WC. Neoverataline A and B, two antifungal alkaloids with anovel carbon skeleton from Veratrumtaliense. Tetrahedron 2003; 59: 5743-5747.
- Alkhalaf M. Resveratrol-induced growth inhibition in MDAMB-231 breast cancer cells is associated with mitogenactivatedprotein kinase signaling and protein translation. Eur J Cancer Prev 2007; 16: 334-341.
- WU SL, SUN ZJ. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J Gastroentero2004; 20: 3048-3052.
- Choi HY, Chong SA, Nam MJ. Resveratrol induces apoptosis in human SK-HEP-1 hepatic cancer cells. Cancer Genomics Proteomics 2009; 6: 263-268.
- Yaseen A, Chen S, Hock S, Rosato R, Dent P, Dai Y, Grant S. Resveratrol sensitizes acute myelogenous leukemia cells to histonedeacetylase inhibitors through reactive Oxygen species-mediated activation of the extrinsic apoptotic pathway. MolPharmacol 2012; 82: 1030-1041.
- Wang GQ, Cheng XY, Wu CF, Zhang L, Gao DL, Zhang YH, Chen KS, Zhao ZH. Effect of resveratrol on mRNA expression of human programmed cell death 5 in nude mice transplanted tumor tissue of human gastric adenocarcinoma. Journal of Zhengzhou University (Medical Sciences) 2010; 45: 569-572.
- Trincheri NF, Nicotrag, Folloc. Resveratrol induces cell death in colorectal cancer cells by a novel pathway involving lysosomalcathepsin D. Carcinogenesis 2007; 28: 922-931.
- Adam G, Schreiber K, Tomko J, Vassova A, Verazin, einneuesveratrum-alkaloid mit 22,26-iminocholestan-gerüst. Tetrahedron 1967; 23: 167-171.
- Tang J, Li HL, Shen YH, Jin HZ, Yan SK, Liu RH, Zhang WD. Four new germine esters from Veratrum dahuricum. Helv.Chim.Acta 2007; 90: 769-775.
- Ling Y, Bao YY, Zhu LL, Zheng JH, Cai SQ, Xiao Y. Study on the chemical constituents of Taraxacum. Chinese Pharmaceutical Journal 1997; 32: 584-586.
- Liao LP, Li P. Compounds from leaf of Ilex purpureaHassk. Journal of China Pharmaceutical University 2004; 35: 205-206.
- Hanama F, Tahara S, Mizutani J. Antifungal stress compounds from Veratrumgrandiflorum leaves treated with cupric chloride. Phytochemistry 1992; 31: 3005- 3007.
- Usia T, Iwata H, Hiratsuka A, Watabe T, Kadota S, TezukaY. Sesquiterpenes and Flavonol Glycosides from Zingiberaromaticum and Their CYP3A4 and CYP2D6 Inhibitory Activities. Nat. Prod. 2004; 67: 1079-1083.