ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2014) Volume 25, Issue 4
Anti-inflammatory action of Ginkgo Biloba leaf polysaccharide via TLR4/NF-?b signaling suppression.
1Department of Cell Biology, College of Basic Medical Sciences, Jilin University, Changchun, Jilin 130021, P.R. China
2Department of Biotechnology, College of Life Science, Changchun Teaching University, Changchun, Jilin 130002, P.R. China
- *Corresponding Author:
- R. Fei
Department of Cell Biology
College of Basic Medical Sciences, Jilin University
126 Xinmin Street, Changchun, Jilin 130021
P.R. China
Accepted July 25 2014
Ginkgo biloba is a valuable herb that has been used as traditional medicine for thousands of years. Extract of Ginkgo biloba leaves possesses several clinical beneficial effects such as antiinflammatory property. In this study, we investigated the effect of purified polysaccharides from Ginkgo biloba leaf (P-PGBL) on inflammatory reaction induced by lipopolysaccharides (LPS) in RAW264.7 mouse macrophage cells and the potential underlying mechanisms. The results showed that P-PGBL treatment significantly inhibited the LPS-induced protein and mRNA expression of Toll-like receptor 4 (TLR4) in a dose-dependent manner. P-PGBL treatment downregulated the nuclear factor-κB (NF-κB)p65 protein expression in LPS-stimulated RAW264.7 macrophage cells. Meanwhile, the levels of pro-inflammatory cytokines, such as IL-1β and IL-6 were suppressed by P-PGBL. In conclusion, P-PGBL exerts an anti-inflammatory effect by inhibiting TLR4/NF-κB signaling pathway, thereby lowering production of inflammatory cytokines.
Keywords
Anti-inflammatory; Ginkgo biloba; polysaccharides; Toll-like receptor 4 (TLR4); nuclear factor-κB (NF-κB)
Introduction
Inflammation is a physiological response against invading pathogens and tissue injury. This process involves different cell types, such as macrophages, mast cells, dendritic cells, granulocytes and the innate lymphocytes [1]. It has been reported that the Toll-like receptor 4 (TLR4) signaling pathway plays an important role in the development of inflammation [2]. TLR4 was the first of the human TLRs to be identified, which responds to lipopolysaccharides (LPS) from Gram-negative bacteria. Recognition of lipid A by TLR4 leads to the production of a wide range of immune stimulatory cytokines and chemokines, such as interleukins (ILs), interferon and tumor necrosis factor (TNF)-α [3,4]. Researches indicated that inflammatory responses contribute to various diseases, for example, excessive inflammation can lead to further tissue injury and fibrosis [5,6], and the lack of an inadequately controlled inflammatory response can lead to chronic inflammation, cancer [7], diabetes [8], cardiovascular disease [9] and autoimmune-mediated diseases [10].
Ginkgo biloba is a valuable herb that has been used as “medicine food homology” medicine for thousands of years [11]. In these traditional formulations, Ginkgo biloba leaves provide relief from asthma and cough symptoms, and can alleviate neurological problems due to its neuroprotective effects on the brain [12,13]. Over the past couple of decades, researches concerning its medicinal effects and biological activities have revealed various effects of Ginkgo biloba extract in treating diseases, such as Alzheimer’s [14], cardiovascular diseases [15], cancer [16], stress and memory loss [17]. Polysaccharides of Ginkgo biloba leaves are active components with multiple pharmacological functions, for instance, oxyradical and hydroxyl radical clearance effects, anti-aging properties, antitumor activities, and immunomodulatory effects [18,19]. Rough polysaccharides have been reported to have anti-inflammatory effects [20]. In our previous work, we have purified the rough polysaccharides through gel chromatography, and obtained a water-soluble purified Ginkgo biloba leave polysaccharides (P-PGBL) with uniform molecular weight. Preliminary study showed that the P-PGBL could inhibit inflammation in vivo [21].
In the present study, we investigated the antiinflammatory activity of P-PGBL in LPS-stimulated mouse macrophage RAW264.7 cells by examining changes in levels of pro-inflammatory cytokines. Fur thermore, the expression of Toll-like receptor 4 (TLR4) and downstream molecules of TLR4 pathway were examined to investigate the potential mechanisms involved in the effects of P-PGBL.
Materials and Methods
Materials
RAW264.7 cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Dulbecco's Modified Eagle Medium (DMEM) both with and without phenol red, Hanks' balanced salt solution (HBSS), and phosphate buffered saline (PBS) were from Gibco (Carlsbad, USA). Fetal bovine serum (FBS), and LPS from Escherichia coli serotype 0111:B4 were obtained from Sigma (St. Louis, USA). FITC conjugated goat anti-rabbit IgG, rabbit anti TLR4 polyclonal antibody, NF-κB(p65) rabbit polyclonal antibody were purchased from Abcam (Cambrige, England). All other chemicals and reagents were of HPLC grade to be made in China.
Isolation and purification of Ginkgo biloba leave polysaccharides
Ginkgo biloba leaves were collected from Dandong, Liaoning Province, China. The P-PGBL were extracted and purified from fresh Ginkgo biloba leaves according to our previous methods [21]. The crude powder was fractionalized by gel chromatography on a Sephadex G-75 column (4 cm×80 cm) and collected by an automatic fraction collector (BS-100A, Shanghai Huxi Analysis Instrument Inc., Shanghai, China). Then the fraction with the highest peak on the distribution curve was dialyzed, concentrated, and freeze-dried to obtain P-PGBL. P-PGBL purity was measured by gel chromatography on a Sephadex G-75 column (1.5 cm×95 cm) and HPLC, respectively. All gel chromatography was monitored with the phenol-sulfuric acid method. The molecular weight of P-PGBL was calculated according to the calibration curve obtained by using various standard dextrans. Nucleotides and proteins in P-PGBL were inspected by a UV spectrum assay. Optical rotation was measured with a WZZT1 polarimeter (Shanghai Physical Optics Instrument Inc., Shanghai, China). The polysaccharide component was determined by gas chromatography (GC).
Cell culture and treatment
RAW264.7 mouse macrophage cells were cultured in DMEM medium supplemented with 10% FBS, glutamine, and antibiotics at 37°C under 5% CO2. Cells at 80–90% confluency were centrifuged at 120×g at 4°C for 10 min and cell concentration was adjusted to (2× 106) cells/ml, where by the cell viability always was more than 90%. A total of 50 μl of cell suspension was seeded into a tissue culture grade 96-well plate (4× 105 cells/well) and incubated for 2 h at 37°C, 5% CO2 for cells attachment. Then, cells were stimulated by using 1 μg/ml of LPS with or without the presence of P-PGBL tested at the final volume of 100 μl/well. Cells were further incubated at 37°C, 5% CO2 for 24 h for use.
Cell viability
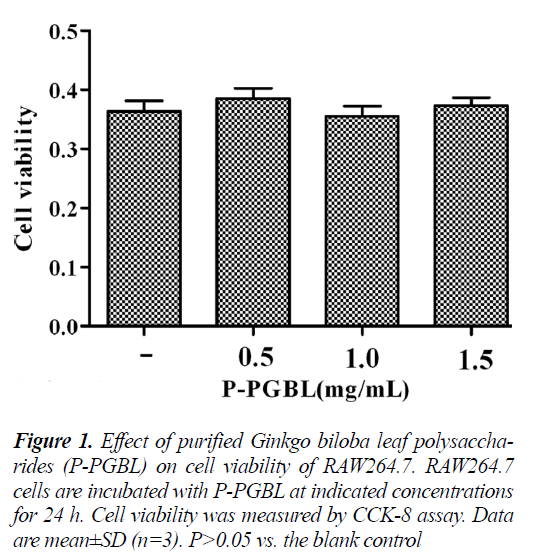
The viability of cells was measured using Cell Counting Kit-8 (CCK-8) assay. Briefly, 4 × 104 cells/well RAW264.7 cells were plated in 96-well plates, incubated for 2h, then treated with different concentrations (0.5, 1.0 and 1.5 mg/ml) of P-PGBL for 24 h. Equal volume of medium was used as blank control. After treatment, cells were incubated with 10 μl of CCK-8 for another 4 h in dark. The absorbance at 450 nm was recorded using a PR 4100 microplate reader (Bio-Rad Inc., Hercules, CA, USA).
Analysis of cytokines using ELISA assay
IL-1β and IL-6 concentrations in the cell culture supernatant were detected using the enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instruction, and the results were expressed in pg/ml of protein. All of the analyses were performed in triplicate.
Quantitayive real time-(q)PCR
Total RNA was extracted from RAW264.7 cells using TRIzol Reagent (Invitrogen). And mRNA was reversed transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (TransGen Biotech Inc., Beijing, China) according to the manufacturer’s instructions. Equal amounts of cDNA were submitted for PCR in the presence of SYBR Green I reagent and forward and reverse primers using the Bio-Rad iQ5Quantitative PCR System. The specific primers of TLR4 were (sense) 5’- AAG GCA TGG CAT GGC TTA CAC-3’, and (antisense) 5’-GGC CAA TTT TGT CTC CAC AGC-3’. The NF-κB primers were (sense) 5’-CCC AAA CCT TGG CAT CCT G-3’ and (antisense) 5’-CCG AAC AAC ACT CAA ATC C-3’. The IL-6 primers were (sense) 5’-CAG AGA TAC AAA GAA ATG AT-3’ and (antisense) 5’-GTC CCA ACA TTC ATA TTG TC-3’. The IL-1β primers were (sense) 5’-GCC TCG TGC TGT CGG ACC CAT AT-3’ and (antisense) 5’- TCC TTT GAG GCC CAA GGC CAC A -3’. PCR were subjected to one cycle of 94°C for 10 min and then 40 cycles (94°C for 30 s, 59°C for 30 s and 72°C for 30 s).
Western blot analysis
After treatment with LPS (1 μg/ml) in the presence or absence of P-PGBL, RAW264.7 cells were washed with cold PBS. Total proteins of cells were extracted with cell lysis buffer (50 mM Tris–HCl pH 8.0, 120 mM NaCl, 0.5% NP-40, 1 mM PMSF), and 40 μg of protein extract was separated by 10% SDS-PAGE. The extracted protein was transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad), blocked with 5% nonfat milk in TBS-Tween buffer 7 (0.12M Tris–base, 1.5M NaCl, 0.1% Tween-20) for 1h at room temperature and incubated with the appropriate antibody overnight at 4°C. Later this was incubated with horseradish peroxidase conjugated secondary antibody for 30min at room temperature. The bound antibody was detected with peroxidase-conjugated antimouse antibody (1:10,000) followed by chemiluminescence (ECL System) and exposed by autoradiography. The protein bands were quantified by the average ratios of integral optic density following normalization to the GAPDH.
Immunofluorescent microscopy
RAW 264.7 cells were plated at 5 × 104 cells/well in 24- well chamber slides (Falcon). For immunostaining, cells were washed twice with cold phosphate-buffered saline, fixed in 4 % paraformaldehyde for 20 min, permeabilized with 0.3 % Triton X-100 for 15 min, and blocked with 10 % normal goat serum for 30 min. Slides were incubated for 1h at 37°C with primary antibody at a 1:100 dilution, washed, and then incubated for 1h with FITC-conjugated IgG (Molecular Probes) at a 1:100 dilution as secondary antibodies. Images were obtained with an Olympus IX71 microscope (Japan).
Statistical analysis
Statistical analyses were carried out with SPSS 13.0 software (SPSS Inc., IL, USA). Data were analyzed by a oneway ANOVA, followed by Student’s two-tailed t test for comparison between two groups, and data were presented as mean ± SD. P < 0.05 was statistically significant difference.
Results
Cell viability and cytotoxicity
The effect of P-PGBL on cell viability was evaluated using the Cell Counting Kit-8 assay. As depicted in Fig. 1, following a 24h treatment, P-PGBL at concentrations ranging from 0.5 to 1.5 mg/ml had no effect on RAW264.7 cell viability. Hence these concentrations of PPGBL were considered suitable for further assays.
P-PGBL down regulates pro-inflammatory cytokines in LPS-stimulated RAW 264.7 cells
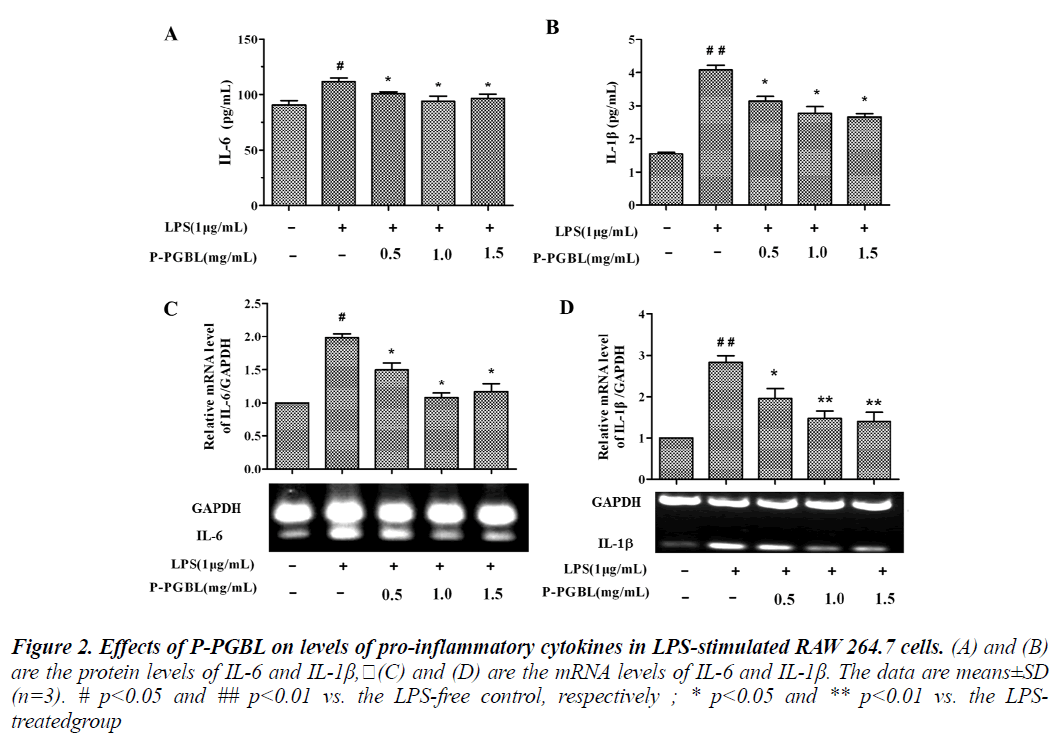
Pro-inflammatory cytokines play a key role in the process of inflammation and inflammation associated diseases. Studies have shown that, the levels of pro-inflammatory cytokine IL-6 increased significantly in inflammation associated diseases such as rheumatoid arthritis and cancer patients [22,23]. We examined the effects of P-PGBL on the expression of pro-inflammatory cytokines IL-6 and IL-1β. Induction of RAW264.7 cells into an inflammatory state by treatment with LPS resulted in markedly increase in IL-6 and IL-1β production. In ELISA assay, P-PGBL in the range of 0.5 to 1.5 mg/ml concentrations inhibited the expression of IL-6 and IL-1β markedly (Fig. 2A and B).
Figure 2: Effects of P-PGBL on levels of pro-inflammatory cytokines in LPS-stimulated RAW 264.7 cells. (A) and (B) are the protein levels of IL-6 and IL-1β,(C) and (D) are the mRNA levels of IL-6 and IL-1β. The data are means±SD (n=3). # p<0.05 and ## p<0.01 vs. the LPS-free control, respectively ; * p<0.05 and ** p<0.01 vs. the LPStreatedgroup
Furthermore, the levels of IL-6 and IL-1β mRNA were examined by qPCR. As shown in Fig. 2C and D, LPS induced the mRNA of IL-6 and IL-1β, by comparison, PPGBL attenuated the LPS-induced IL-6 and IL-1β mRNA levels in a dose-dependent manner. These results suggest that P-PGBL may reduce the expression of proinflammatory cytokines IL-6 and IL-1β.
Suppression of LPS-stimulated TLR4 expression by PPGBL in RAW 264.7 cells
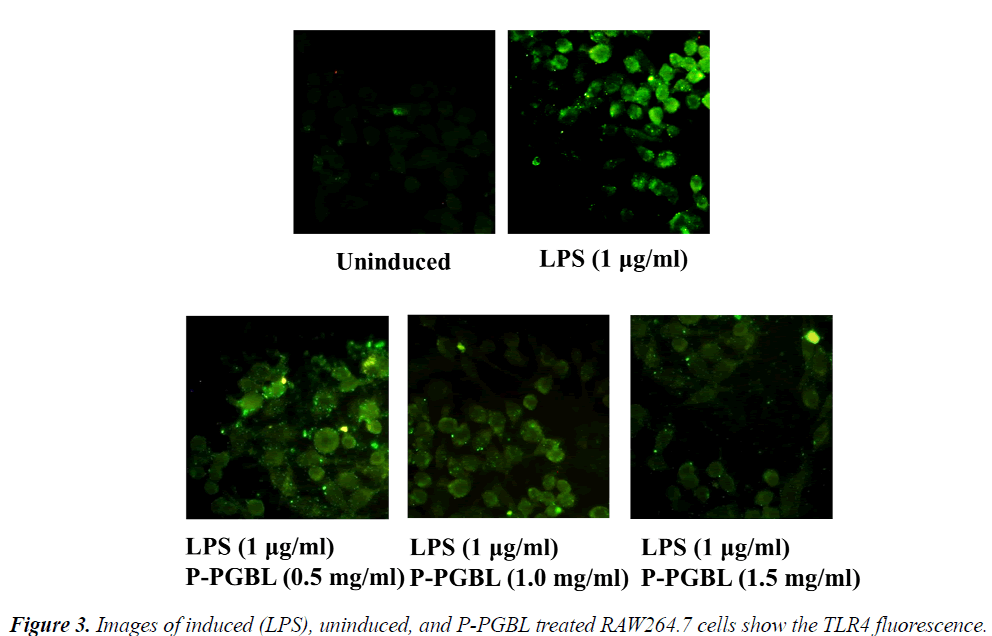
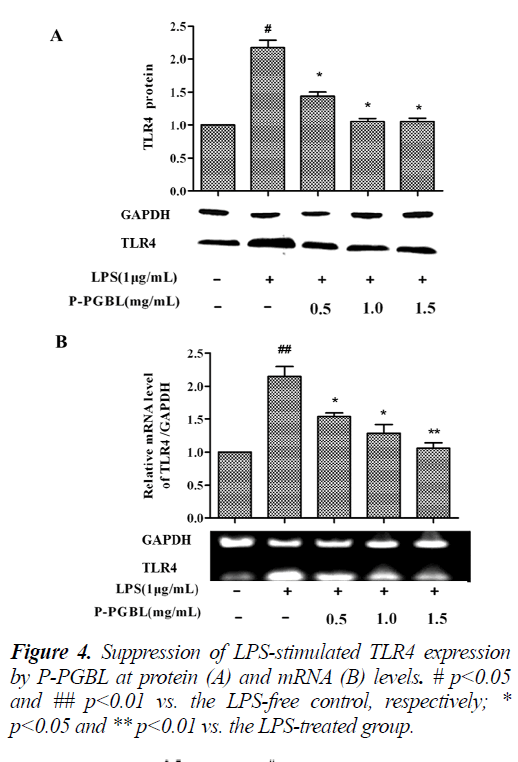
The TLR4 signaling plays an important role in the development of inflammation. We further investigated the effects of P-PGBL on TLR4 expression in LPS-stimulated macrophage. Western blot analysis showed that the level of TLR4 protein was decreased by treatment with PPGBL, as compared with the LPS-stimulated group (Fig. 3 and 4A). Moreover, treatment with P-PGBL also downregulated the mRNA level of TLR4 in cells (Fig. 4B).
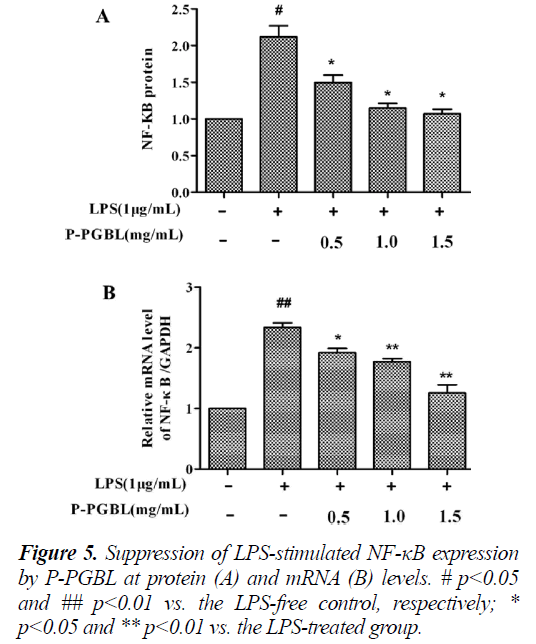
Suppression of LPS-stimulated NF-κB p65 by P-PGBL in RAW 264.7 cells
In order to investigate the effect of P-PGBL on TLR4 signal pathways, we measured the expression of NF-κB in inflammatory cells by western blot and qPCR. As shown in Fig. 5, both the results of western blot analysis and qPCR demonstrated that, the protein and mRNA level of NF-κB induced by LPS in RAW cells decreased significantly (P < 0.05) in a dose dependent manner when treated with P-PGBL. These data together with the above results suggested that P-PGBL might provide protection from LPS-induced inflammation through the suppression of TLR4/NF-κB pathways.
Discussion
In this study, we investigated the anti-inflammatory activity of P-PGBL in LPS-stimulated mouse macrophage RAW264.7 cells, and examined its effect on the expression of TLR4 and NF-κB, which is a key molecule of TLR4 signaling pathway. We demonstrated that P-PGBL could inhibit LPS-induced production of inflammatory cytokines. Moreover, treatment with P-PGBL reduced the expression of TLR4 and NF-κB in LPS-stimulated macrophages, suggesting that P-PGBL might reduce proinflammatory cytokines through down regulating TLR4 and NF-κB.
A moderate amount of TLR4 expression can regulate the body's immune function, but the over-expression of excessive TLR4 can cause inflammatory diseases. Studies have found excessive expression of TLR4 in cancer and chronic inflammation diseases such as liver cirrhosis, tuberculosis and atherosclerosis patients [24]. Our results showed that, LPS can significantly increase the expression of TLR4 in RAW264.7 cells, consistent with previous reports, while treatment with P-PGBL could decrease the expression of TLR4 in a dose-dependent manner. These results suggested that, the anti-inflammatory activity of P-PGBL closely associate with TLR4, and TLR4 is an important target of P-PGBL.
NF-κB is considered as a master switch in the regulation of inflammation and immunity. As a transcription factor, NF-κB controls an array of pro-inflammatory genes involved in the inflammatory signaling cascade. Therefore, we speculated that the anti-inflammatory effect of PPGBL in response to LPS-induced inflammation correlated with NF-κB. To our expected, level of NF-κBp65 expression reduced in LPS-stimulated macrophages after treated with P-PGBL. These results demonstrated that PPGBL could inhibit the expression of inflammatory cytokines IL-6 and IL-1β through inhibiting the TLR4/ NF-κB pathway.
Conclusions
P-PGBL has good anti-inflammatory action. One of the underlying mechanisms may be that P-PGBL can inhibit the expression of inflammatory cytokines IL-6 and IL-1β by suppressing the TLR4/NF-κB signal pathways. This study not only provides the experimental evidence for the development of anti-inflammatory natural Ginkgo biloba polysaccharide drugs.
Acknowledgments
This research was financially supported by the "Twelfth Five-Year" Science and Technology Research Project of The Education Department of Jilin province (no. 2014- 270), and the Natural Science Fund of Changchun Teaching College.
References
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodula- tory mast cells: negative, as well as positive, regulators of immunity. Nature Rev Immunol 2008; 8:478-486.
- Ospelt C, Gay S. TLRs and chronic inflammation. Int J Biochem Cell Biol 2010; 42: 495-505.
- Kim EY, Moudgil KD. Regulation of autoimmune in- flammation by pro-inflammatory cytokines. Immunol Lett 2008;120: 1-5.
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32: 593-604.
- López-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progres- sion. EMBO Mol Med 2009; 6-7: 303-314.
- Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl 2010; 119: S22-26.
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013; 13: 759-771.
- Donath MY, Dalmas É, Sauter NS, Böni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab 2013; 17: 860-872.
- Gómez-Guerrero C, Mallavia B, Egido J. Targeting inflammation in cardiovascular diseases. still a ne- glected field? Cardiovasc Ther 2012; 30: e189-197.
- Marzano AV, Tedeschi A, Polloni I, Crosti C, Cugno M. Interactions between inflammation and coagulation in autoimmune and immune-mediated skin diseases. Curr Vasc Pharmacol 2012; 10: 647-652.
- Chan PC, Xia Q, Fu PP. Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Envi- ron Sci Health C Environ Carcinog Ecotoxicol Rev 2007; 25: 211-244.
- Singh B, Kaur P, Gopichand, Singh RD, Ahuja PS. Bi- ology and chemistry of Ginkgo biloba. Fitoterapia 2008; 79: 401-418.
- Ryu EY, Park AJ, Park SY, Park SH, Eom HW, Kim YH, et al. Inhibitory effects of Ginkgo biloba extract on inflammatory mediator production by Porphyromonas gingivalis lipopolysaccharide in murine macrophages via Nrf-2 mediated heme oxygenase-1 signaling path- ways. Inflammation 2012; 35: 1477-1486.
- Ude C, Schubert-Zsilavecz M, Wurglics M. Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients. Clin Pharmacokinet 2013; 52: 727-749.
- Tunali-Akbay T, Sener G, Salvarli H, Sehirli O, Yarat A. Protective effects of Ginkgo biloba extract against mer- cury(II)-induced cardiovascular oxidative damage in rats. Phytother Res 2007; 21: 26-31.
- Tsai JR, Liu PL, Chen YH, Chou SH, Yang MC, Cheng YJ, et al. Ginkgo biloba extract decreases non-small cell lung cancer cell migration by down regulating me- tastasis-associated factor heat-shock protein 27. PLoS One 2014; 9: e91331.
- Mohanta TK, Tamboli Y, Zubaidha PK. Phytochemical and medicinal importance of Ginkgo biloba L. Nat Prod Res 2014; Feb 5.
- Chen Q, Liu TJ. Studies on immunomodulatory and antineoplastic activities of polysaccharide isolated from Ginkgo biloba leaf (GBLP). Pharmacol Clin Chin Mat Med 2003; 19: 18-19.
- Hăncianu M, Pavelescuz M, Miron A, Aprotosoaie AC, Grigorescu E, Lupuşoru C, et al. Polysaccharides frac- tion isolated from ginkgo biloba folium: immunophar- macological properties. Rev Med Chir Soc Med Nat Iasi 2007; 111: 1070-1073.
- Song LY, Ma WX, Yu RM, Kan QM, Yao XS. Studies on the biological activities of polysaccharides from the cell cultures and the leaves of Ginkgo Biloba. Chin J Biochem Pharm 1999; 20: 278-280.
- Fei R, Fei Y, Zheng S, Gao YG, Sun HX, Zeng XL. Purified polysaccharide from Ginkgo biloba leaves in-hibits P-selectin-mediated leucocyte adhesion and in-flammation. Acta Pharmacol Sin 2008; 29: 499-506.
- Müller N, Döring F, Klapper M, Neumann K, Schulte DM, Türk K, et al. Interleukin-6 and Tumour Necrosis Factor-α differentially regulate lincRNA transcripts in cells of the innate immune system in vivo in human subjects with rheumatoid arthritis. Cytokine 2014; 68: 65-68.
- Wang SW, Sun YM. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer. Int J Oncol 2014; 44: 1032-1040.
- Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll- like receptors: emerging therapeutics? Nat Rev Drug Discov 2010; 9: 293-307.