ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 1
Analysis of eponemycin (a'├¤' epoxyketone) analog compound from Streptomyces hygroscopicus subsp Hygroscopicus extracts and its antiplasmodial activity during Plasmodium berghei infection
1Laboratory of Parasitology, Faculty of Medicine, Universitas Brawijaya, Jl Veteran, Malang, Indonesia
2Medical Study Program, Faculty of Medicine, Universitas Brawijaya, Jl Veteran, Malang, Indonesia
3Master Program in Biomedical Sciences, Faculty of Medicine, Universitas Brawijaya, Jl Veteran, Malang, Indonesia
4Eijkman Institute for Molecular Biology, Jl Diponegoro 69, Jakarta 10430, Indonesia
- *Corresponding Author:
- Rivo Yudhinata Brian Nugraha
Faculty of Medicine Universitas Brawijaya Indonesia
Accepted on May 12, 2016
Background & objectives: The greatest challenge in reducing the high mortality and morbidity of malaria is due to the emerging incidence of artemisinin resistance. We analyzed eponemycin analog in Streptomyces hygroscopicus subsp Hygroscopicus extract and its anti-plasmodial activity in P. berghei.
Methods: Isolate of S. hygroscopicus was macerated using ethyl acetate: International Streptomyces Project 4/ISP4 medium (1:1 v/v) and analyzed using Thin Layer Chromatography (TLC) and High Performance Liquid Chromatography (HPLC). Plasmodium berghei-infected mice were grouped into a group of non-treated control and three groups treated with various dosage of S. hygroscopicus (130, 580, and 2600 μg/kgBW for 5 days).
Results: TLC analysis showed a spot with Refractory factor (Rf) 0.7. HPLC demonstrated 3.768% and 5.796% Dyhidroeponemycin in two samples. The degree of parasite on Day 3-6 (compared to Day 1) was significantly lower when a series of dosages of 130, 580, and 2600 μg/kgBW were given. Treatment with this compound at the dosage of 2600 μg/kgBW reduced the degree of parasite on almost all days. Histological examination of the spleen showed morphological improvement in all treated groups.
Conclusion: Eponemycin analog in crude metabolite extracts of S. h. Hygroscopicus is potential candidate for a new antimalarial drug.
Keywords
S. h. Hygroscopicus, Eponemycin, Malaria, Spleen.
Introduction
The spread of antimalarial drug resistance for treatment and prevention of malaria has caused major problems in malaria control programs. Resistance to antimalarial agents has been reported for human’s plasmodial species including Plasmodium falciparum, P. vivax, and P. malariae [1]. Artemisinin resistance was reported in P. falciparum in South East Asia including Cambodia, Myanmar, Thailand, and Vietnam. Between 2001 and 2007, the proportion of patients that were parasitemic on day 3 after treatment with either artemetherlumefantrine or artesunate-mefloquine exceeded 10% in western part of Cambodia. The proportion of patients with parasitemia on day 3 after treatment with dihydroartemisininpiperaquine (DHAP) increased from 26% to 45% between 2008 and 2010 [2]. Plasmodium vivax has developed resistance against sulfadoxine-pyrimethamine (SP) and chloroquine (CQ) in Indonesia, Papua New Guinea, Timor-Leste, and Oceania [1].
Along with the slow progress of effective malaria vaccine discovery, the findings of new antimalarial drugs remain the main priority. Ubiquitin-Proteasome System (UPS) is a system consisted of ubiquitin and multi-subunit protease complex in all eukaryotic cells. The UPS has a central role for quality control of gene expression and gives response toward oxidative stress. 20S proteasome as well as ubiquitin gene are expressed during Plasmodium life cycle [1]. Proteasome has important role on all stage of Plasmodium during its life cycle [1,2]. 20S proteasome has an active CP SU (Core Protein Sub-Unit) with caspase-like activity, trypsin-like, and chymotrypsin-like respectively.
Many studies have reported potent effects of variety proteasome inhibitors against each stage of Plasmodium. Parasites treated with proteasome inhibitor demonstrated inhibition in DNA replication phase due to the high number of ubiquitinated protein. Proteasome of Plasmodium has been shown its existence indirectly by using its inhibitor. Previous study showed that proteasome inhibitor for malaria, lactacystin, inhibited liver and blood-stage Plasmodium growth prior to DNA synthesis [3]. A similar effect was reported for MLN-273 and the activity of this compound against the proteasome was confirmed by demonstrating the accumulation of ubiquitinated proteins in treated parasites [4].
Our previous study demonstrated that the crude metabolite extract of Streptomyces hygroscopicus subsp. Hygroscopicus contained eponemycin, a potential candidate for a new antimalarial drug through inhibition of UPS function of the parasite and can cause stress to P. berghei. Metabolite extract of S. hygroscopicus Hygroscopicus (Actinomycetes family) at dosage of 2600 mg/kgBW caused morphological changes and damage to P. berghei possibly through inhibition of UPS as revealed by the increased accumulation of ubiquitinated protein of P. berghei [5]. This research is aimed to explore the effect of eponemycin analog contained in S. hygrocopicus Hygroscopicus isolated from Indonesia and examine its activity as an anti-malarial agent.
Materials and Methods
Research design
The eponemycin analog contained in S. hygroscopicus was analyzed by Thin Layer Chromatogaphy (TLC) and High Performance Liquid Chromatography (HPLC). The study was done to reveal the activity of crude metabolite extracts of S. hygroscopicus in inhibiting the growth of P. berghei in BALB/C mice and examine the spleen morphology by histology.
Samples were divided into a control group where mice were infected with P. berghei without any treatment. The treatment groups consisted of mice that were infected with P. berghei and treated intra-peritoneally with metabolite extract of S. hygroscopicus subsp. hygroscopicus at the dosage of 130 μg/ kgBW, 580 μg/kgBW, and 2600 μg/kgBW for 5 days respectively). Each group consisted of five male mice with the age-range between 8-12 weeks. The degree of parasitaemia in the infected mice was calculated on the first day of malaria infection until the last treatment. Histopathology of the spleen was examined.
Exploration of eponemycin analog contained in Streptomyces hygroscopicus Hygroscopicus
Streptomyces hygroscopicus Hygroscopicus medium: International Streptomyces Project (ISP4) medium consisted of 10 g soluble starch, 1 g of K2HPO4, 1 g of MgSO4.7H20, 1 g of NaCl, 2 g of (NH4)2SO4, 2g of CaCO3, 1 ml of trace salt solution (consisted of 0.1 g of Fe2SO4.7H20, 0.1 g of ZnSO4.7H2O, 0.1 g of MnCl2.4H2O in distilled water), 20 g agar, 1 L distilled water. The medium pH was adjusted to between 7.0-7.4 and autoclaved at 121°C for 15 minutes [6].
Inoculation, fermentation, and metabolite extraction: About 25.8 × 106 bacteria were inoculated in 50 ml ISP4 medium in the Erlenmeyer glass and were incubated for 7 days at 28°C in shaking incubator. Fermentation was carried out in 2 ml of bacteria suspension were mixed in 50 ml of ISP4 medium in an Erlenmeyer bottle. The mixture was incubated for 7 days at 28°C in shaking incubator. The fermented medium was mixed with ethyl-acetate 1:5 (v/v) and shaken for 1 hour, then deposited in a separate funnel for 4 hours. Bacterial metabolite was bound in solvent in the upper side at the ethyl-acetate phase. Water phase was discarded and the ethyl-acetate phase was collected. Subsequently, the mixture was heated at 80-90°C waterbath until a dried powdered metabolite extract was obtained [7].
Thin layer chromatogaphy (TLC) preparation
Streptomyces hygroscopicus extract in the form of powder was dissolved in methanol. TLC chamber was coated by filter paper on each side. Silica plate was cut into 2 × 10 cm. Sample was spotted on paper as much as 30 mL with silica capillary tube. Sample adequacy checking was done by examining the spot appearance under 254 nm UV light. Selected eluent was inserted into the chamber and then silica plate was placed into the chamber in an upright position and allowed the the eluent was absorbed until the upper limit. Observation was done under 254 nm UV light. To detect the desired compound, silica plate was sprayed with anisaldehide then heated to observe the color changes. Further reading was done under 365 nm UV light [8].
High performance liquid chromatography (HPLC) preparation
Preparation of dihydroeponemycin standard: One mg of dihydroeponemycin standard was dissolved in 5 mL flask with 200 ppm dichloromethane: MeOH (98: 2) eluent. Standard solution was titrated with 6.25; 12.5; and 25 ppm dihydroeponemycin concentration and then 20 μL was injected into the HPLC.
Sample preparation of Streptomyces hygroscopicus extract: Two and a half mg of sample was placed in a 15 mL conical tube. The sample was mixed with 3 ml dichloromethane: methanol (98: 2) eluent. Solution was subjected to ultrasonication for 5 minutes, and put in a 5 mL volumetric flask, placed to mark the eluent, and filtered with Millex 0.45 μm. Twenty μL of the solution was injected into the HPLC.
Experimental study
Healthy BALB/C male mice (8-12 weeks) weighing 30-40 grams were purchased from the Gadjah Mada University were used. Mice were cared in cages 30 × 30 cm with 4-5 mice per cage. Mice were given standard food and drink every day. The study has been approved by the Ethics Commission of the Faculty of Medicine, Universitas Brawijaya.
Preparations and inoculation of P. berghei
Parasites were obtained from Biomedical Laboratory, Universitas Brawijaya. Erythrocyte pellet infected with P. berghei stored in liquid nitrogen tank at -135°C was thawed and subjected to centrifugation at 2000 rpm for 5 minutes. Pellets were washed twice in RPMI medium and diluted as needed for inoculation to donor mice. After parasitemia of donor mice reached more than 10%, blood was collected through cardiac puncture. Parasite infection was done to the experimental mice intraperitoneally at 107 parasites in 0.2 ml [9].
Metabolite extracts of S. hygroscopicus subsp. Hygroscopicus treatment
Crude metabolite extracts of S. hygroscopicus subsp. Hygroscopicus was dissolved in DMSO using sonicator. Dilution was made with RPMI medium to make concentration of 130, 580, and 2600 μg/kgBW per 200 μl. Treatment was given by injecting a total of 200 μl metabolite extracts of S. hygroscopicus subsp. Hygroscopicus in mice intraperitoneally once daily for five days.
Parasitemia evaluation
Thin blood smear from mouse tail was made to evaluate parasitemia after each treatment once daily for six days. Thin blood smear on the object glass was methanol fixated. Slides were stained with 20% Giemsa for 20 minutes and washed by distilled water. Observation was done under 1000x magnification. Degree of parasite was counted based on total infected erythrocytes per 1000 erythrocytes.
Histopathology examination of the spleen
On the sixth day after five-day treatment, mice were sacrificed using inhaled-chloroform. Intra-cardiac blood and spleen were collected. Spleen histopathology was prepared using paraffin block method. Cross sectional slices were made in 6-8 μm thickness. Sections were performed from end to middle-end side of the spleen. The sections were stained using Hematoxylin-Eosin (HE). Spleen morphology was observed under microscope at 100x and 400x magnification.
Data analysis
Data analysis was performed using SPSS 16. Factorial repeated-measures ANOVA test was used with p value <0.05. Spleen histopathology was described morphologically.
Results
Thin layer chromatography (TLC) of S. hygroscopicus extract
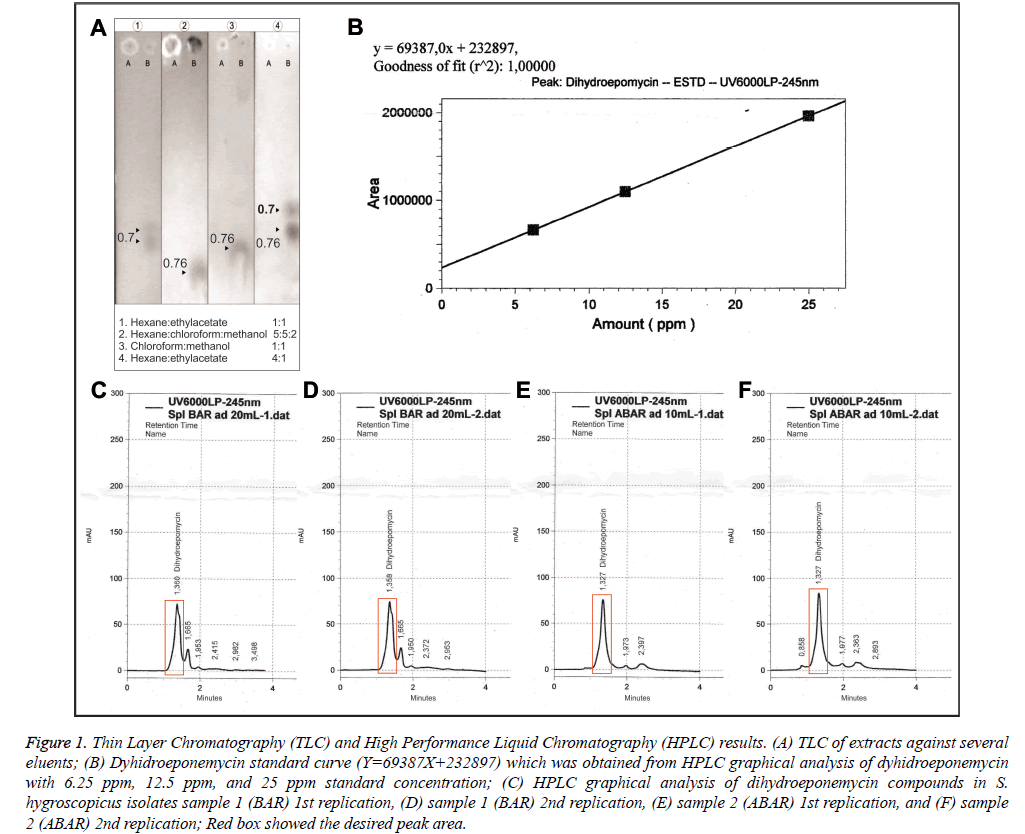
The sample used was fermentation medium (Figure 1A.A) and methanol dissolved fraction (Figure 1A and 1B). Thin Layer Chromatography (TLC) was performed using four kinds of mobile phase eluent to yield an optimal characterization of the extract content. TLC mobile phase eluents used were in the Figure 1A. Refractory factor (Rf) was obtained by dividing the stains distance from the spot with total distance (Figure 1A). Rf was used as determinants of a compound against eluent polarity.
Figure 1: Thin Layer Chromatography (TLC) and High Performance Liquid Chromatography (HPLC) results. (A) TLC of extracts against several eluents; (B) Dyhidroeponemycin standard curve (Y=69387X+232897) which was obtained from HPLC graphical analysis of dyhidroeponemycin with 6.25 ppm, 12.5 ppm, and 25 ppm standard concentration; (C) HPLC graphical analysis of dihydroeponemycin compounds in S. hygroscopicus isolates sample 1 (BAR) 1st replication, (D) sample 1 (BAR) 2nd replication, (E) sample 2 (ABAR) 1st replication, and (F) sample 2 (ABAR) 2nd replication; Red box showed the desired peak area.
In Figure 1A, Thin Layer Chromatography (TLC) 1 used hexane and ethyl acetate at 1: 1 in a total of 10 ml. Second eluent (TLC) 2 used hexane, chloroform, and methanol at 5: 5: 2 respectively in a total of 12 ml. Third eluent (TLC) 3 used chloroform and methanol at 1: 1 in a total of 10 ml. Fourth eluent (TLC) 4 used hexane and ethyl acetate at 4: 1 in a total of 10 ml. The sample used was the result of the fraction dissolved in methanol and fermented medium. Based on the TLC 1 results, it was showed a visible staining on fraction with Rf value of 0.70. Thin Layer Chromatography (TLC) 2 demonstrated staining on the fraction with Rf value of 0.76. Thin Layer Chromatography (TLC) 3 showed two stainings with Rf value of 0.76 on a fraction with a very visible staining and the medium with the same Rf on fraction with unclearly visible staining. TLC 4 had two stainings with Rf values of 0.70 and 0.76 with clearly visible staining.
High performance liquid chromatography (HPLC) of S. hygroscopicus extract
High Performance Liquid Chromatography (HPLC) measurements used dihydroeponemycin as standard. HPLC test samples were extracted from S. hygroscopicus subsp Hygroscopicus. The theoretical basis of compound quantification was based on the measurement of the peak height or peak area. Determination of compound concentration used peak area or peak height plotted against the substance concentration used [10]. Analysis of Dyhidroeponemycin standard provided a peak height and the peak area of standard compound. Concentration of standard solution was presented in the form of a Dyhidroeponemycin standard solution curve (Figure 1B) and was used to determine the concentration of eponemycin substances in the Streptomyces hygroscopicus extract which had been divided into two samples (samples 1 and 2) with 2 replications (Figures 1C-1F). After HPLC results analysis on sample 1 and sample 2 data, peak area were obtained in Table 1.
| No | Sample Code | Weight (gr) | End Volume (mL) | Result | Result (%) | ||
|---|---|---|---|---|---|---|---|
| Peak Area | ppm | ||||||
| 1. | Sample 1 (BAR) | 0.0025 | 20.0 | Rep-1 | 727411 | 7.13 | 5.796 |
| Rep-2 | 743243 | 7.36 | |||||
| Mean | 735327 | 7.25 | |||||
| 2. | Sample 2 (ABAR) | 0.0025 | 10.0 | Rep-1 | 864775 | 9.11 | 3.678 |
| Rep-2 | 877133 | 9.28 | |||||
| Mean | 870954 | 9.20 | |||||
BAR: sample with short storage time; ABAR: sample with long storage time; ppm: part per million; Rep: replication.
Table 1. Dyhidroeponemycin Analysis Results in Streptomyces hygroscopicus Extract Sample
Table 1 showed a peak area differences between samples 1 (BAR) and 2 (ABAR) that will be meaningful to the eponemycin compounds concentration in the extract. Higher peak area was obtained in sample 2. Table 1 also presented the results of analysis and calculation of the percentage of eponemycin compounds in each sample. Dyhidroeponemycin concentration, which was an eponemycin analog, in sample 1 and 2 were at 5.796% and 3.678% respectively.
The degree of parasite
This study was true experimental with randomized post-test control group design. There were two independent variables: groups (control, 130, 580, and 2600 μg/kgBW doses) and days (day 1-6) of treatment. Analysis was performed using factorial repeated-measures ANOVA with p value=0.05. This analysis evaluated main effect of groups, main effect of days, interaction, and simple contrasts of variables.
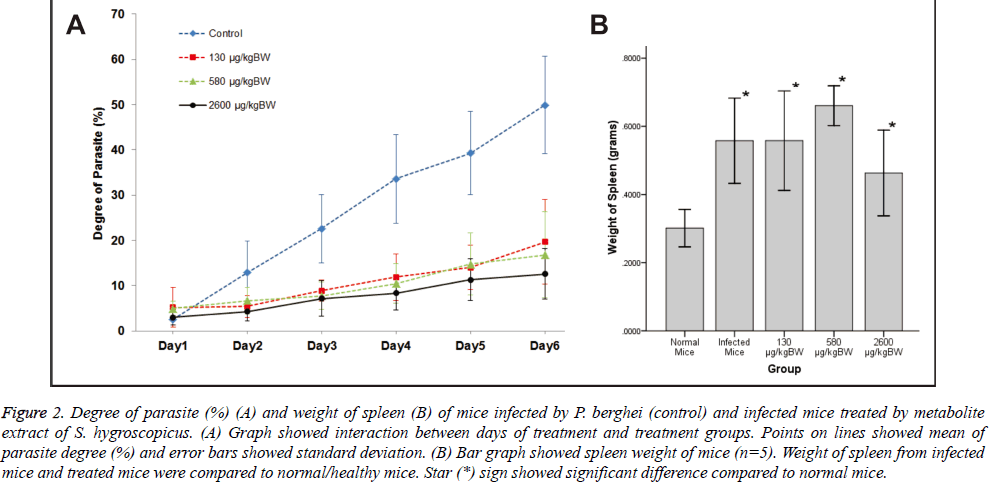
There was significant main effect of groups of treatment on the degree of parasite, F (3, 12)=20.75, p<0.001. In contrast, all treatment groups [130 μg/kg BW F(1,4)=20.25, p=0.011, r=0.91; 580 μg/kgBW F (1,4)=36.07, p=0.004, r=0.95; and 2600 μg/kg BW F (1,4)=28.36, p=0.008, r=0.94] were significantly lower compared to base category (control group). Meanwhile, the main effect of days of treatment [F (1.51, 6.06)=86.96, p<0.001] significantly increased the degree of parasite day by day due to parasite infection.
Days of treatment had different effect on degree of parasite depending on which groups of treatment given [F (15,60)=9.62, p<0.001]. To explain this interaction, contrasts were performed comparing all groups of treatment to their baseline (control group) and all days of treatment to their baseline (day 1). Based on the interaction graph (Figure 2A), the degree of parasite on day 2-6 (compared to day 1) were significantly suppressed [day 2 F (1,4)=11.82, p=0.026, r=0.86; day 3 F (1,4)=26.01, p=0.007, r=0.93; day 4 F (1,4)=32.40, p=0.005, r=0.94; day 5F (1,4)=35.60, p=0.004, r=0.95; and day 6 F(1, 4)=23.24, p=0.009, r=0.92 respectively] when 130 μg/kg BW doses of treatment given. Meanwhile, degree of parasite on day 2 (compared to day 1) was not significantly suppressed [F (1,4)=7.23, p=0.055] by 580 μg/kgBW doses. However, degree of parasite on day 3-6 (compared to day 1) were significantly suppressed [day 3 F (1,4)=28.35, p=0.006, r=0.94; day 4 F (1,4)=31.63, p=0.005, r=0.94; day 5 F (1,4)=27.02, p=0.007, r=0.93; and day 6 F(1, 4)=23.24, p=0.009, r=0.92 respectively] when 580 μg/kgBW doses of treatment given. Moreover, degree of parasite on day 2-6 (compared to Day 1) were significantly suppressed [day 2 F (1,4)=10.50, p=0.032, r=0.85; day 3 F (1,4)=21.35, p=0.010, r=0.92; day 4 F (1,4)=29.13, p=0.006, r=0.94; day 5 F (1,4)=27.93, p=0.006, r=0.94; and day 6 F (1,4)=43.25, p=0.003, r=0.96 respectively] when 2600 μg/kgBW doses of treatment given.
Figure 2: Degree of parasite (%) (A) and weight of spleen (B) of mice infected by P. berghei (control) and infected mice treated by metabolite extract of S. hygroscopicus. (A) Graph showed interaction between days of treatment and treatment groups. Points on lines showed mean of parasite degree (%) and error bars showed standard deviation. (B) Bar graph showed spleen weight of mice (n=5). Weight of spleen from infected mice and treated mice were compared to normal/healthy mice. Star (*) sign showed significant difference compared to normal mice.
Groups of treatment also had different effect on degree of parasite depending on days of treatment (F (15,60)=10.47, p<0.001). To explain this interaction, contrasts were performed comparing two consecutive days of treatment and all groups of treatment to their baseline (control). Based on the interaction graph (Figure 2A), doses of 130 and 2600 μg/kgBW could significantly suppress the degree of parasite [F (1,4)=11.82, p=0.026, r=0.86 and F (1,4)=10.49, p=0.032, r=0.85 respectively] than control on day 2 of treatment (compared to day 1) in contrast to 580 μg/kg BW doses of treatment [F (1,4)=7.23, p=0.055, r=0.80]. Doses of 130 and 580 μg/kg BW could significantly suppress the degree of parasite [F (1,4)=11.64, p=0.027, r=0.86 and F (1,4)=14.26, p=0.020, r=0.88 respectively] than control on day 3 of treatment (compared to day 2), inverse to 2600 μg/kg BW doses of treatment [F (1,4)=7.44, p=0.053, r=0.81]. All doses could significantly suppress the degree of parasite [130 μg/kg BW F (1,4)=9.95, p=0.034, r=0.84; 580 μg/kgBW F (1,4)=16.52, p=0.015, r=0.90; and 2600 μg/kg BW F(1,4)=58.61, p=0.002, r=0.97] than control on day 4 of treatment (compared to day 3). However, all doses could not significantly suppress the degree of parasite [130 μg/kg BW F (1,4)=1.64, p=0.270, r=0.54; 580 μg/kg BW F (1,4)=0.15, p=0.720, r=0.19; and 2600 μg/kgBW F (1,4)=1.06, p=0.361, r=0.46] than control on day 5 of treatment (compared to day 4). Only 2600 μg/kgBW doses could significantly suppress the degree of parasite [F (1,4)=12.53, p=0.024, r=0.87] than control on day 6 of treatment (compared to day 5).
Spleen weight and histopathology
Mice spleens were weighted after euthanized. Weight of spleen was compared between 5 groups (each n=5): normal mice, infected mice, 130 μg/kgBW, 580 μg/kgBW, and 2600 μg/ kgBW dose group (Figure 2B). There was significant difference among these groups with p-value= 0.001. From LSD Post Hoc Test, there was significant increase in spleen weight of infected mice and treated mice compared to normal mice (p>0.05). However, there was no significant difference of spleen weight between infected mice and treated mice (p>0.05). Among treated group, only the highest dose (2600 μg/kgBW) showed significant decreased of spleen weight compared to 580 μg/kgBW dose (p=0.009).
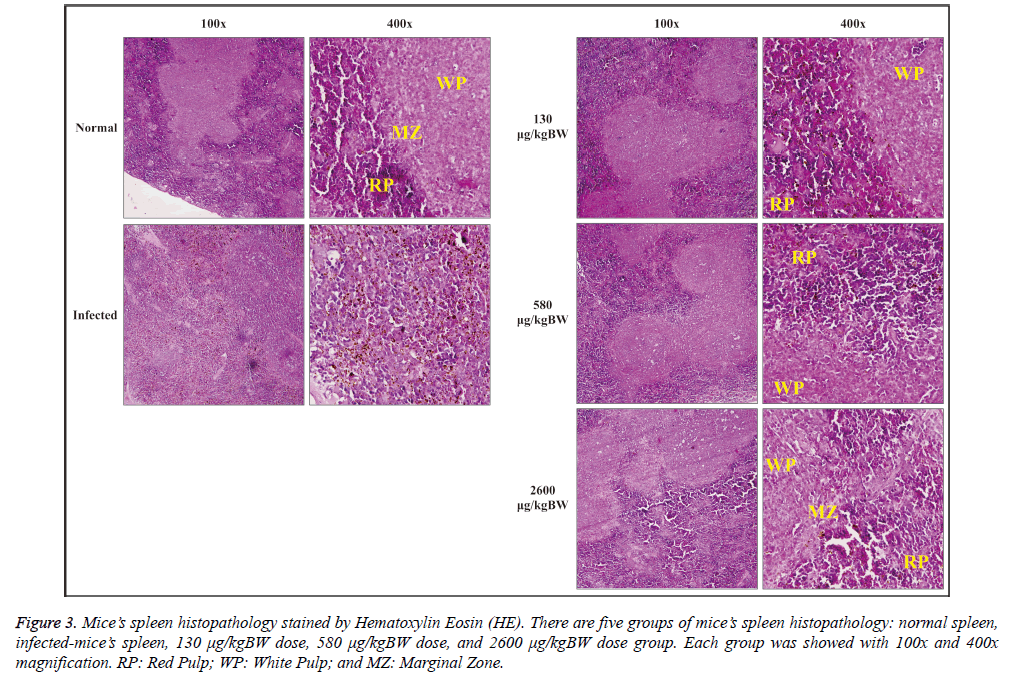
Histopathological observation of spleen was done by staining the slide with hematoxylin-eosin (HE). Spleen histopathology was observed under a microscope with 100x and 400x magnification as seen at Figure 3. Normal spleen morphology on negative control group showed a clear spleen histological structure, i.e. the red pulp and white pulp which consists of three parts: periarteriolar lymphoid sheath (PALS), follicular, and marginal zone. Red pulp consisted of splenic cords and venous sinuses structure. Penicillar arterial and arteriolar were also clearly seen. Marginal zone appeared to form a barrier between the white pulp and red pulp accompanied by macrophages around them.
Figure 3: Mice’s spleen histopathology stained by Hematoxylin Eosin (HE). There are five groups of mice’s spleen histopathology: normal spleen,infected-mice’s spleen, 130 μg/kgBW dose, 580 μg/kgBW dose, and 2600 μg/kgBW dose group. Each group was showed with 100x and 400x magnification. RP: Red Pulp; WP: White Pulp; and MZ: Marginal Zone.
The spleen histopathology of malaria-infected mice (Figure 3) showed that the spleen zone differences were not apparent between the red pulp, white pulp, and marginal zone. Marginal zone seemed to disappear. Red pulp sinusoids appeared distended and degraded hemozoin was detected
Histopathological observation of spleen in the treatment group generally gave morphological improvement effect in line with the increased dose given (Figure 3). The structure of the red pulp, white pulp, and marginal zone were not apparent in the 130 μg/kgBW treatment group accompanied by red pulp sinusoid distention and hemozoin deposition. Meanwhile, the spleen structure (the red pulp, white pulp, and marginal zone) of 580 μg/kgBW treatment group could be distinguished. 2600 μg/kgBW treatment group had a similar structure compared to the healthy control group with minimal accumulation of hemozoin pigment.
Discussion
Thin layer chromatography (TLC)
Thin Layer Chromatography (TLC) 1 showed there were still fluoresce under UV light and two stains that appear on the sample fractions after anisaldehide spraying spaced somewhat close together. Therefore, there was still material that did not rise from the spot. Repetition TLC2 and TCL3 with different eluent were aimed to raise the material. TLC 4 conducted with same eluent but with different compositions (hexane is more). TLC 4 was obtained two same stains with a little distance away. It can be concluded there were approximately 2-3 kind of compounds from the stain appeared.
Based on the results, TLC1, that had appeared stains with Rf value of 0.70 in ethyl acetate and hexan eluent as much as 1: 1 in 10 ml, has a very dominant compound in existing stain. These results are consistent with studies of Ho, 2008, which did TLC on eponemycin analog compounds (compounds standard), on 1: 1 eluent with results Rf 0.70. It can be concluded that the fraction (extract) that is used in this study contains eponemycin analog compounds (eponemycin and derivatives) based on the TLC existing results by comparing with TLC study results using the eponemycin analog standard above [11,12].
High performance liquid chromatograhy (HPLC)
This study used HPLC analysis with two samples consisted of sample 1 (BAR), result of S. hygrocopicus extracts that were stored no longer than 1 week, and sample 2 (ABAR), result of S. hygrocopicus extracts that have been stored between 2-3 weeks. This sample division aimed to determine whether there were compound concentrations differences in the eponemycin extract with short and long storage time. Eponemycin compound concentration was measured at λ 245 nm. Selection of wavelenght and type of eluent was based on some literature that analyzed eponemycin compounds in the extract. Standard solution used for eponemycin compounds sample measurement in HPLC is Dyhidroeponemycin standard solution which is eponemycin analogous compound [12]. Differences in concentrations above indicated that the compound may undergo concentration or level changes caused by storage time, and because of other factors that can affect the concentration of a compound. The result of this exploration is important in the utilization of Streptomyces hygroscopicus extract in antimalarial drug development in order to calculate the effective dose of extract that effect on Plasmodium growth.
Anti-plasmodial activity of S. hygroscopicus extract
The P. falciparum genome possesses genes predicted to encode all 14 subunits of a eukaryote-type 20S proteasome [13]. The 20S proteasome is a cylindrical assembly of 28 individual proteins arranged as 4 stacked rings, each comprising 7 subunits. The two inner rings consist of seven different β subunits each, three of which are catalytically active (β1, β2, and β5). Rings of seven different subunits on each side flank the two β subunit rings to form a barrel-like structure. According to their proteolytic mechanism, proteasomes are classified as N-terminal nucleophilic hydrolases (Ntnhydrolases) or threoninepeptidases [14].
As shown on Figure 2A, higher dosage of S. hygroscopicus extract results on lower degree of parasite particularly for 580 and 2600 μg/BW doses. According to days of treatment,degree of parasite increases significantly on day 3-day 6. These results are actually affected by control group which has steady escalation on degree of parasite. Although all of groups have increasing tendencies on degree of parasite, there is parasite growth inhibition affected by extracts treatment. Although there is a parasite degree’s decreasing tendency observed on the graph, differences among treatment doses don’t give significant differences on parasite degree per day. However while comparing parasite degree on two consecutive days, treatment dose variant effects will be more apparent. Generally, all doses can suppress the degree of parasite in the treatment groups than control. Particularly, 2600 μg/kgBW dose can suppress degree of parasite on almost all days of treatment and the only one on the last day of treatment.
The pattern of the antiplasmodial compounds is consistent with the fact that the proteasome is present throughout the whole asexual cycle and correlates with the function of the proteasome, which is a key regulator in housekeeping functions, such as cell cycle progression, in most eukaryotes. This is an advantage over most established antimalarial drugs, which are active against distinct stages intraerythrocytic development only. Gametocytocidal activity is also rarely observed among established drugs and is increasingly considered important, especially for malaria elimination efforts. In this study, killing of trophozoite stage is similar to the killing mechanism of the proteasome inhibitor MG132. This is in accordance with previous findings on the antimalarial effect of proteasome inhibitors, which describe parasite growth inhibition prior to DNA synthesis [1,15].
Another proteasome inhibitors that demonstrate activity against all blood stages, including rings [15,16] and gametocytes [16,17] as well as hepatic stages [4] had revealed in different studies. Activity against early and late blood stages is advantageous because it offers rapid parasite clearance during infection, which is especially important in severe malaria. Gametocytocidal activity is particularly important when elimination of malaria is the goal. Most registered drugs and drug candidates have low or no activity against gametocytes. Recent study introduces peptido sulfonyl fluorides (PSF) as a new class of compounds with antiplasmodial activity. PSF target the plasmodial proteasome and act on all asexual stages of the intraerythrocytic cycle and on gametocytes. PSF has a great potential for further development as preclinical candidate compounds with improved species-specific activity and less toxicity [18].
Crude metabolite extracts S. hygroscopicus subsp. Hygroscopicus that contained eponemycin. Eponemycin and its analog are one of natural peptide in epoxyketonegroup which is used in this research. Until date, peptide α’β’- epoxyketone is known as the most specific and potent natural from the first generation of proteasome inhibitors [2]. Epoxomicin, one of epoxyketone group, was extensively studied and known to have antiplasmodial activity on both asexual and asexual stage of P. falciparum. Epoxomicin also effectively kill all stages of intraerythrocytic parasites but do not affect the viability of human and mouse cell lines [19].
Another epoxyketone group which is epoxomicin analog, Carfilzomib, can reduce parasite load in P. berghei infected mice without host toxicity [20]. No study reports eponemycin or dyhidroeponemycin effect on the growth of plasmodium. Epoxomicin that have been developed and intensively characterized might carry a chemically reactive ‘‘warhead’’ at the C-terminus to deactivate the proteasome (e.g. epoxyketone, aldehyde, boronic acid) intermittently or even irreversibly. However, recently reversible high-affinity inhibitors of this kind have been reported as well [21], which do not undergo a covalent attachment. Furthermore, by appropriate tailoring of the chemical structures, subsite-specificity for the CP-SUs b1, b2, or b5 was achieved [22].
Considering the rapid DNA replication during erythrocytic trophozoite stage where there is distinct up-regulation in the expression in the mid/late trophozoite to early schizont stages coinciding with the active replication window [23]. Transcriptome analysis has also shown that during late trophozoite to schizont stage transformation, there is upregulation of ubiquitin-proteosomal degradation genes signed its importance pathway in Plasmodium [19]. Although this study results on inhibition of parasite growth instead of parasite immediate killing, viability test has revealed that there is no parasite survive and multiply [5]. This may due to eponemycin’s irreversible binding.
Eponemycin is one of epoxyketone group which is known as the most specific and potent proteasome inhibitors. Proteasome inhibitors can demonstrate activity against all blood stages as well as hepatic stages that revealed in different studies. This study reports eponemycin in S. hygroscopicus metabolite extract inhibits the growth of Plasmodium. Higher dosage of extract results on lower degree of parasite particularly for 580 and 2600 μg/kg BW doses. Particularly, 2600 μg/BW dose can suppress degree of parasite on almost all days of treatment and the only one on the last day of treatment.
Effect of S. hygroscopicus extract on spleen weight and histologic
Malaria causes disturbance of immune responses with major changes on spleen architectural. The size of spleen increased, caused by cells hyperplasia that occurs in the red pulp and white pulp region. Red pulp widened and became a hematopoiesis side. Splenic cord and sinus filled by monocytes and macrophages containing pigment, as well as infected and normal erythrocytes. Marginal zone transiently disappear [20,24]. Such changes may affect the strength of the immune response to malaria. T cells cannot migrate into the B cells zone so that inhibit its activation, plasma cells are short-lived, and remain in the spleen which contribute to slow responses of antibody, and impaire of antibody affinity maturation [25].
In this study, histological examination of the spleen showed morphological improvement in all S. hygroscopicus treated groups marked by clear structure of the red pulp, white pulp, and marginal zone of the spleen. Although this metabolite extract can improve infected spleen morphology, it is not affected the weight of infected spleen. All treated group has significant increase of spleen weight compared to normal mice. However, the highest dose/2600 μg/kgBW still can significantly decrease the weight of infected spleen compared to 580 μg/kgBW dose.
References
- Kreidenweiss A, Kremsner PG, Mordmüller B. Comprehensive study of proteasome inhibitors against Plasmodium falciparum laboratory strains and field isolates from Gabon. Malar J 2008;7:187.
- Aminake M, Arndt H, Pradel G. The proteasome of malaria parasites : A multi-stage drug target for chemotherapeutic intervention. Int J Parasitol Drugs Drug Resist 2012;2:1-10.
- Gantt SM, Myung JM, Briones MRS, Li WD, Corey EJ, Omura S. Proteasome Inhibitors Block Development of Plasmodiumspp . Proteasome Inhibitors Block Development of Plasmodium spp. Antimicrob Agents Chemother 1998;42: 2731-2738.
- Lindenthal C, Weich N, Chia Y, Heussler V, Klinkert M. The proteasome inhibitor MLN-273 blocks exoerythrocytic and erythrocytic development of Plasmodium parasites. Parasitology 2005; 131:37-44.
- Rivo Y, Alkarimah A, Ramadhani NN, Cahyono AW, Laksmi DA, Winarsih S. Metabolite extract of Streptomyces hygroscopicusHygroscopicus inhibit the growth of Plasmodium berghei through inhibition of ubiquitin – proteasome system. Trop Biomed 2013;30:291-300.
- Shepherd MD, Kharel MK, Bosserman MA, Rohr J. Laboratory maintenance of Streptomyces species. CurrProtocMicrobiol 2010;1:1-10.
- Sharma H, Parihar L. Antifungal activity of extracts obtained from Actinomycetes. J Yeast Fungal Res 2010;1:197-200.
- Sin N, Meng L, Auth H, Crews CM. Eponemycin analogues: syntheses and use as probes of angiogenesis. Bioorg Med Chem 1998;6:1209-1217.
- Moll K, Ljungström I, Perlmann H, Scherf A, Wahlgren M. Methods in Malaria Research. 5th ed. Manassas: Malaria Research and Reference Reagent Resource Center (MR4) American Type Culture Collection; 2004.
- Kupiec. Quality-Control Analytical Methods: High-Performance Liquid Chromatography. Int J Pharm Compd 2004;8: 223-227.
- Berger D. Organic Chemistry Laboratory Manual. Bluffton; 2011.
- Ho YK. A Novel Class of Immunoproteasome Catalytic Subunit LMP2 Inhibitor and Its Therapeutic Potentials in Cancer. University of Kentucky, 2008.
- Gille C, Goede A, Schlöetelburg C, Preißner R, Kloetzel PM, Göbel UB. A comprehensive view on proteasomal sequences: Implications for the evolution of the proteasome. J MolBiol 2003;326: 1437-1448.
- Löwe J, Stock D, Jap B, Zwickl P, Baumeister W, Huber R. Crystal Structure of The 20S Proteasome from The ArchaeonT. acidophilum at 3.4 A Resolution. Science (80- ) 1995;268:533-539.
- Reynolds JM, El Bissati K, Brandenburg J, Günzl A, Mamoun C Ben. Antimalarial activity of the anticancer and proteasome inhibitor bortezomib and its analog ZL3B. BMC ClinPharmacol 2007;7:13.
- Aminake MN, Schoof S, Sologub L, Leubner M, Kirschner M, Arndt HD. Thiostrepton and derivatives exhibit antimalarial and gametocytocidal activity by dually targeting parasite proteasome and apicoplast. Antimicrob Agents Chemother 2011; 55: 1338-1348.
- Czesny B, Goshu S, Cook JL, Williamson KC. The proteasome inhibitor epoxomicin has potent Plasmodium falciparumgametocytocidal activity. Antimicrob Agents Chemother 2009;53: 4080-4085.
- Tschan S, Brouwer AJ, Werkhoven PR, Jonker AM, Wagner L, Knittel S. Broad-spectrum antimalarial activity of peptidosulfonyl fluorides, a new class of proteasome inhibitors. Antimicrob Agents Chemother 2013;57: 3576-3584.
- Sinha A, Sarkar S. Ubiquitin-Proteasome System- a target to control pathogenic protozoa. Formatex2013:764-773.
- Rungruang T, Chaweeborisuit P, Klosek SK. Effect of malaria infection and dexamethasone on spleen morphology and histology. Southeast Asian J Trop Med Public Health 2010;41: 1290-1296.
- Blackburn C, Gigstad KM, Hales P, Garcia K, Jones M, Bruzzese FJ. Characterization of a new series of non-covalent proteasome inhibitors with exquisite potency and selectivity for the 20S beta5-subunit. Biochem J 2010;430: 461-476.
- Britton M, Lucas MM, Downey SL, Screen M, Pletnev AA, Verdoes M. Selective inhibitor of proteasome’s caspase-like sites sensitizes cells to specific inhibition of chymotrypsin-like sites. ChemBiol 2009;16: 1278-1289.
- Mitra P, Deshmukh AS, Dhar SK. DNA replication during intra-erythrocytic stages of human malarial parasite Plasmodium falciparum. CurrSci 2012;102: 725-740.
- Cesta MF. Normal structure, function and histology of the thymus. ToxicolPathol 2006;34: 504-514.
- Del Portillo H, Ferrer M, Brugat T, Martin-Jaular L, Langhorne J, Lacerda MVG. The role of the spleen in malaria. Cell Microbiol 2012;14:343-355.