ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 4
Acinetobacter species-associated infections and their antibiotic susceptibility profiles in Malaysia
Nazmul MHM*, Jamal H, Fazlul MKK.
Department of Microbiology, Faculty of Medicine, Universiti Teknologi MARA, Shah Alam, Malaysia.
- Corresponding Author:
- Mohammad Nazmul Hasan Maziz
Department of Microbiology Faculty of Medicine Universiti Teknologi MARA Shah Alam, 40450 Selangor Malaysia
Accepted date: July 11 2012
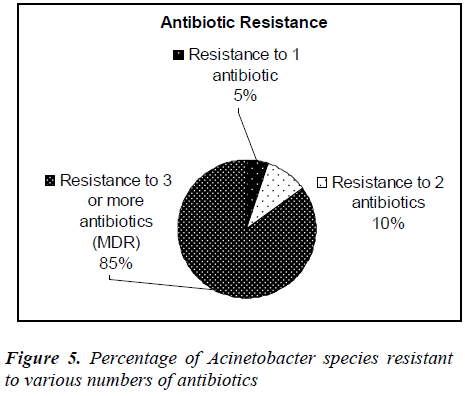
Forty clinical isolates of Acinetobacter species were collected from Selayang hospital, Selangor, Malaysia. Thirty-nine percent of the isolates were identified from respiratory tract followed by urine (19%) and pus (18%). All the isolates were re-identified and confirmed as Acinetobacter species in our laboratory. The antibiotic susceptibility profiles of all the isolates were determined using Kibry-Bauer disk diffusion method as recommended by CLSI. Aminoglycosides (gentamicin) was found to be the most active antimicrobial agent with 47.5% susceptibility followed by amikacin (45%) and 30% to the beta-lactams (imipenem, ceftazidime). Meropenem showed the maximum resistance (92.5%) followed by piperacillin (77.5%) and ampicillin (75%). It was also found that 5% of the Acinetobacter strains were resistant to one antibiotic, 10% strains were resistant to two antibiotics and 85% strains were multidrug resistant. Acinetobacter spp isolated from respiratory tract, urine and pus showed the highest rate of multidrug resistance.
Keywords
Antibiotic susceptibility, Multi-drug resistance, Acinetobacter species.
Introduction
The genus Acinetobacter has a long and convoluted taxonomic history. In 1911 Beijerinck, a Dutch microbiologist working in Delft, isolated and described the organism which is now recognised as Acinetobacter [1]. In the nature Acinetobacter spp are widely distributed. Species of the genus Acinetobacter is ubiquitous, free-living and fairly stable in the environment. They are strictly aerobic nonfermentative and Gram-negative bacilli that emerge as significant nosocomial pathogens in the hospital setting and are responsible for intermittent outbreaks. The outbreak of Acinetobacter is much more in the regions where temperature is hot and humid. Pneumonia, septicemia, wound sepsis, urinary tract infection, endocarditis and meningitis are the common infections caused by Acinetobacter spp and is a known nosocomial pathogen causing a wide range of clinical diseases including blood stream infections (BSI).
Acinetobacter species cause infections that are difficult to control due to multi-drug resistance. Acinetobacter species are noted for their intrinsic resistance to antibiotics and for their ability to acquire genes encoding resistance determinants. Foremost among the mechanisms of resistance in this pathogens are the production of betalactamases and aminoglycoside-modifying enzymes [2].
Nevertheless, little knowledge has been gained on tracing the development of antibiotic resistance in Acinetobacter species. However, there are few recent surveillance studies reporting in Malaysia showed multiple-drug resistance of Acinetobacter species and the development of antibiotic resistance in Acinetobacter species [3].
The aim of this study was to assess the current levels of antimicrobial susceptibility and to evaluate the resistance and their biological response towards different antibiotics among the clinical isolates of Acinetobacter species isolated from patients admitted to Selayang Hospital, Malaysia.
Materials and Methods
Forty clinical isolates of Acinetobacter strains were collected from different patients who were admitted to Selayang Hospital, Selangor, Malaysia between January 2010 and June 2010. The Acinetobacter isolates were obtained from different clinical specimens including pus, respiratory fluids, blood, tracheal asp, abdominal fluid, sputum and urine. All the clinically isolated samples were identified as Acinetobacter species by the hospital personnel. We have reidentified all the isolates as Acinetobacter species at our laboratory by the conventional biochemical tests [4] i.e., gram staining, catalase test, oxidase test , motility test, Triple Sugar Iron Assay, citrate test, urease test and indole test etc.
Antibiotic susceptibility testing
The Kirby-Bauer disk diffusion method [5] was performed to determine the antibiotic susceptibility. The antibiotics tested were Gentamicin (10 μg), Impenem (10 μg), Amikacin (30 μg), Piperacillin (100 μg), Ciprofloxacin (5 μg), Ceftazidime (30 μg), Cefoperazone (75 μg), Piperacillin/ Tazobactam (110 μg), Meropenem (10 μg), and Ampicillin (10 μg), respectively. Results of disk diffusion method were interpreted in accordance to the Clinical and Laboratory Standards Institute (CLSI, 2009).
Results
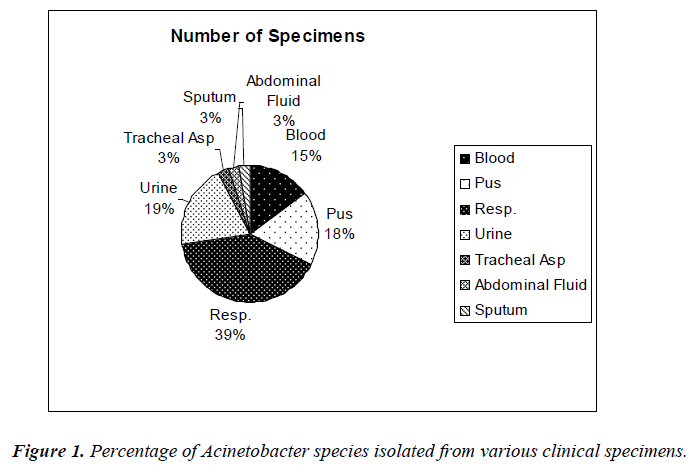
The sources of clinical specimens from patients of Selayang Hospital are shown in Figure 1.
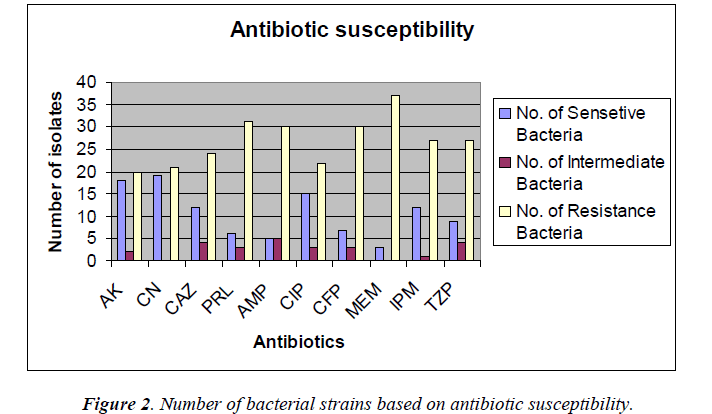
The antimicrobial susceptibility testing revealed that Acinetobacter species strains were resistant to most of the antibiotics tested which are shown in Figure 2.
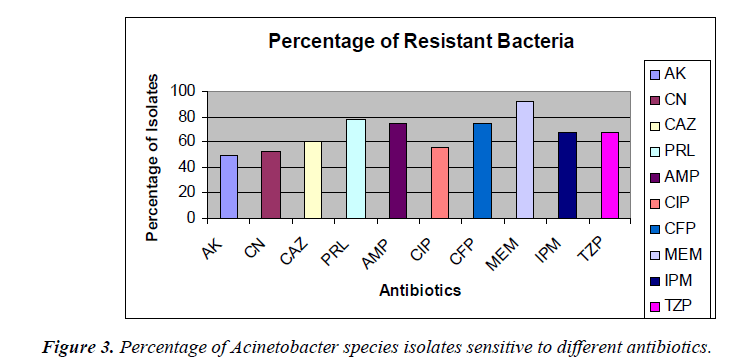
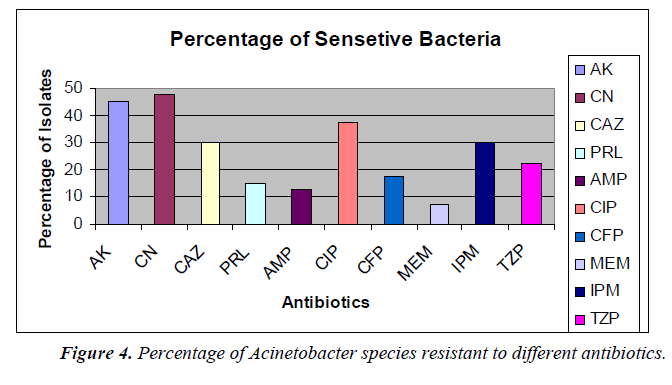
The percentage of sensitivity was; ciprofloxacin (37.5%), imipenem (30%), amikacin (45%), gentamicin (47.5%), ampicillin (12.5%), ceftazidime (30%), meropenem (7.5%), piperacillin (16%), cefoperazone (17.5%), tazobactam 10/ Piperacillin 75 (22.5%) and the percentage of resistance was; ciprofloxacin (55%), imipenem (67.5%), amikacin (50%), gentamicin (52.5%), ampicillin (75%), ceftazidime (60%), meropenem (92.5%), piperacillin (77.5%), cefoperazone (60%), and tazobactam/piperacillin (67.5%) (analyzed from Figure 2), respectively.
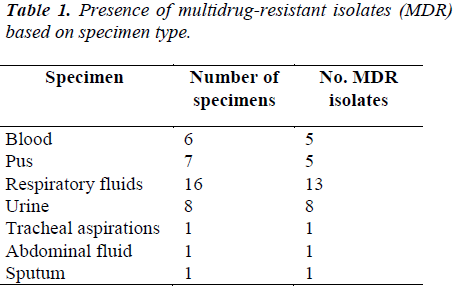
Distribution of the Acinetobacter species isolates according to the specimen type and its correlation to Multi-drug Resistance (MDR) which are shown in Table 1.
Discussion
Acinetobacter species are important nosocomial pathogens, with a rising prevalence of hospital-acquired infection [6]. It was reported that the overall incidence of Acinetobacter species isolation from all infective samples was 9.5% (510 of 5391), indicating its importance as a nosocomial pathogen, since in most cases the patients were symptomatic for sepsis [7]. Acinetobacter spp infections are usually involved with organ systems that have a high fluid content (e.g., respiratory tract, CSF, peritoneal fluid, urinary tract), manifesting as nosocomial pneumonia, infections associated with continuous ambulatory peritoneal dialysis (CAPD), or catheter-associated bacteriuria.
In this present study, it was found that out of 40 clinical Acinetobacter species isolates, 16 strains (40%) were identified from respiratory fluids followed by urine, 8 (20%) cases. Gentamicin was found to be the most effective agents (47.5% sensitivity) followed by amikacin and ciprofloxacin (45% and 37.5%, respectively). Study in India also showed Acinetobacter species were sensitive to amikacin (44.66 %), gatifloxacin and imipenem (33.33%), meropenem and cefaperazone (25%) [8]. Antibiotic susceptibility profiles of Acinetobacter species used at the Universiti Malaya Medical Center (UMMC), Kuala Lumpur, Malaysia were obtained. In descending order of effectiveness, imipenem, amikacin and ciprofloxacin were found to be the most effective against the Acinetobacter species strains [9]. In our studies, the percentages of antimicrobial resistance of the isolates were 92.5% to meropenem, 77.5% to piperacillin, 75% to ampicillin and 50% to amikacin, respectively. From National surveillance on antibiotic resistance of Malaysia, resistance to meropenem increased from 47.7% in 2007 to 54.3% in 2008 and resistance to imipenem was increased from 46.7% in 2007 to 52.9% in 2008. However, this study showed the resistance was 67.5% to imipenem. In this current study, antibiotic resistance to ceftazidime was shown to be 60% but in 2008, resistance to ceftazidime was 49.8%.
It was, however 48.3% in 2007. Resistant to ciprofloxacin was 48.9% of 9101 isolates in 2008 compared to 43.6% of 7479 isolates in 2007. Our study, however, showed 55% to ciprofloxacin and 52.5% to gentamicin where 43.6% of 9265 isolates in 2008 compared to 37.9% of 7886 isolates in 2007. Our study showed 67.5% resistance to piperacillin/tazobactam and imipenem where in 2008 resistant to piperacillin/tazobactam were 55.4% of 6993 isolates compared to 50.9% of 5713 isolates in 2007. In 2008 resistant to ampicillin/sulbactam was found to be 43.2% of 8716 isolates compared to 38% of 7952 isolates in 2007, but current studies showed 75% resistance to ampicillin. Among the 40 clinical isolates of Acinetobacter species tested in our study, many strains were found to be multidrug-resistant (MDR). In this study it was found that, 5% of the Acinetobacter species strains were resistant to one antibiotic, 10% strains were resistant to two antibiotics and 85% were multidrug- resistant (MDR). The resistance to antibiotics of the investigated problematic strains of Acinetobacter species was higher than the mean Acinetobacter species resistance found in Malaysia [7]. In this study, Acinetobacter species isolated from respiratory fluids, pus, blood and urine samples showed the highest rate of multi-drug resistance. Gradually Acinetobacter species is emerging as multidrug- resistant nosocomial pathogen, increasingly involved in hospitalacquired infections but the correlation between the multidrug resistance and the site of infection is not yet known. Risk factor analyses will be useful for further hospital epidemiology studies of Acinetobacter species.
In summary, gentamicin was found to be the most active antimicrobial agent with 47.5% susceptibility followed by amikacin (45%). Meropenem showed the maximum resistance (92.5%) followed by piperacillin (77.5%) and ampicillin (75%). Eighty-five percent of the Acinetobacter species isolates were multidrug-resistant. Acinetobacter species isolated from respiratory tract, urine and pus showed the highest rate of multi-drug resistance (MDR).
Acknowledgements
Valuable suggestion of Professor Amar Chatterjee in structuring the manuscript is gratefully acknowledged. This research was funded by Universiti Teknologi MARA internal DANA Research Grant.
References
- Dijkshoorn L, Nemec A. The diversity of the genus Acinetobacter. In: Gerischer U editors. Acinetobacter molecular biology. Norfolk: Caister Academic Press 2008; 1–34.
- Robert AB, Dora S. Mechanisms of Multidrug Resistance in Acinetobacter species and Pseudomonas aeruginosa. Oxford Journal 2006; 43: S49-S56.
- Nourkhoda S, Reza R, Javad Z, Mohammad YA, Sobhan G, Mohammad R, Ahmed SA, Ali D, Reza M, Fatimah AB. Antimicrobial susceptibility, plasmid profiles, and RAPD-PCR typing of Acinetobacter bacteria. Asian Biomed 2010; 4: 901-911.
- Patrick RM. Manual of Clin Microbiol 2007. 9th ed. Asm Press, Washington D.C., USA.
- Bauer AN, Kirby WMM, Sherris J. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Path 1966; 45: 493-496.
- Lahiri KK, Mani NS, Purai SS. Acinetobacter spp. as nosocomial pathogens: Clinical significance and antimicrobial sensitivity. Med J Armed Forces India 2004; 60: 7-10.
- National surveillance of antibiotic resistance 2008. Retrieved January16, 2011 from http://www.imr.gov.- my/report/nsar.htm.
- George P, Sequiera A. Antimicrobial sensitivity pattern among organisms which were isolated from the endotracheal aspirates of patients with ventilator associated pneumonia. J Clin Diag Res 2010; 4: 3397-3401.
- Misbah S, Yusof MY, Abu BS. Antibiotic susceptibility and REP-PCR fingerprints of Acinetobacter spp. isolated from a hospital ten years apart. J Hosp Infect 2004; 58: 254-261.