ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 10
AB-PAS as a potential alternative for the diagnosis of intestinal metaplasia
Sheng Quan1,2#, Jinping Lin1,2#, Zhinong Jiang3, Xingkang He1,2, Chen Sun1,2, Zhenghua Lin1,2, Tingting Su1,2, Shujie Chen1,2* and Jianmin Si1,2*
1Department of Gastroenterology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, PR China
2Institute of Gastroenterology, Zhejiang University, Hangzhou, Zhejiang Province, PR China
3Department of Pathology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, PR China
#These authors contributed equally to this work
- *Corresponding Authors:
- Jianmin Si
Department of Gastroenterology
School of Medicine
Zhejiang University
PR China - Shujie Chen
Institute of Gastroenterology
Zhejiang University
PR China
Accepted date: March 14, 2017
Background: This study was to investigate the role of AB-PAS in the diagnosis of Intestinal Metaplasia (IM) as compared to HE staining, and to evaluate the relationship between Helicobacter pylori (H. pylori) infection and IM to explore whether IM was a possible pathogenic mechanism of H. pylori in gastric carcinogenesis.
Methods: Patients who suffered from Chronic Atrophic Gastritis (CAG) to Gastric Cancer (GC) were recruited, and the gastric tissues (CAG tissues and cancer tissues) were collected for HE staining, ABPAS and silver staining for detection of IM and H. pylori infection.
Results: Twenty-six patients meeting the inclusion criteria were recruited into the present study and a total of fifty-two slices were available. Thirty-four slices were IM (+) in HE staining, while forty-three slices were IM (+) in AB-PAS. The correlation coefficient between H. pylori infection and IM was about 0.41 (P<0.05).
Conclusions: AB-PAS is superior in the diagnosis of IM to HE staining and IM might be one of pathogenic mechanisms underlying the H. pylori induced gastric carcinogenesis.
Keywords
Intestinal metaplasia, HE staining, AB-PAS, H. pylori infection
Introduction
Gastric Cancer (GC) is one of the leading causes of human cancer-related death and has the second highest incidence and morbidity globally according to the WHO statistics. The overall five-year survival rate of Early Gastric Cancer (EGC) is higher than 90% while the prognosis of Advanced Gastric Cancer (AGC) is still tremendously poor [1]. Therefore, early detection of precancerous lesions is pivotal for the prevention of GC. Intestinal Metaplasia (IM), one of precancerous lesions of GC with the nature of metaplasia [2], can be halted and reversed if interventions are given timely [3]. Hematoxylin- Eosin staining (HE staining) is the most common method used in the diagnosis of IM. However, the procedures and evaluation criteria of HE staining are complex. Thus, to investigate potential staining methods with simple procedures and evaluation criteria is necessary and urgent for the diagnosis of IM.
AB-PAS is a dyes acidic protein secreted by IM cells. Until now, few studies have reported its application in the diagnosis of IM. This study was undertaken to investigate the role of AB-PAS in the diagnosis of IM as compared to HE staining.
There are some risk factors for GC such as family medical history, lifestyle, bile reflux, and Helicobacter pylori (H. pylori) infection [4]. Marshall and Warren originally discovered H. pylori in 1982. H. pylori, prevalent worldwide [5,6], belongs to gram negative, non-spore forming spiral bacteria, and colonizes the human stomach. It is closely related to peptic ulcer and chronic gastritis, which is also one of carcinogenic factors [7-10]. At present, the gastric carcinogenic mechanisms of H. pylori mainly include gene instability, cytokine, immunity, proliferation and apoptosis of gastric epithelial cells [11-14]. It also assumes that H. pylori accounts for the IM of gastric epithelium resulting in GC [15,16], but evidence is still conflicting. Thus, this study was to investigate the role of AB-PAS in the diagnosis of IM as compared to HE staining, and to evaluate the relationship between H. pylori infection and IM to explore whether IM was a possible pathogenic mechanism of H. pylori in gastric carcinogenesis.
Material and Methods
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of Institutional Review Board of Sir Run Run Shaw Hospital and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Clinical samples
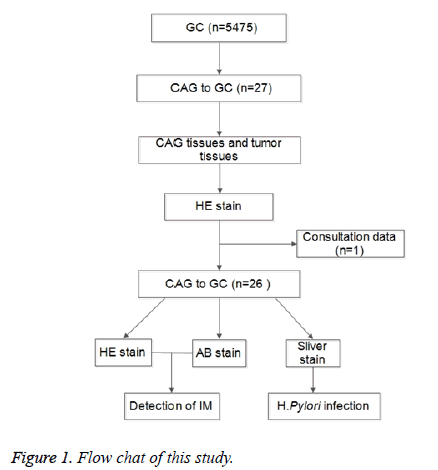
A total of 5475 patients were diagnosed with GC in the Sir Run Run Shaw Hospital from January 1994 to March 2013. Inclusion criteria (diagnosed by pathology) were as follows: (i) a prior history of CAG with or without IM before being diagnosed with GC, (ii) GC was diagnosed based on specimen from the same location where CAG was previously diagnosed. Exclusion criteria were as follows: (i) patients were for the first time diagnosed with GC, (ii) gastric tissues were unavailable (iii) patients was diagnosed with signet-ring cell carcinoma, (iv) other situations not in accordance with the inclusion criteria were present.
Finally, 26 out of 5475 patients met the inclusion criteria and recruited into this study. CAG tissues and cancer tissues were collected by endoscopic biopsy and/or surgery at different time points. Three slices were obtained from each tissue for HE staining and AB-PAS for the evaluation of IM, and for silver staining for the detection of H. pylori infection. The flow chart is shown in Figure 1.
Reagents
Reagents used for different staining were as follows: (i) Hematoxylin solution (H9627-25G, lot#041M0014v) and 1% Eosin Y disodium salt (E6003-25G, lot#MKBP6048V) for HE staining, (ii) Alician Blue 8GX (SIGMA, lot#SLBB1776V) for AB-PAS, and (iii) sliver nitrate (SIGMA S6506-25G, lot#MKBF1627) for silver staining.
Analysis and statistics
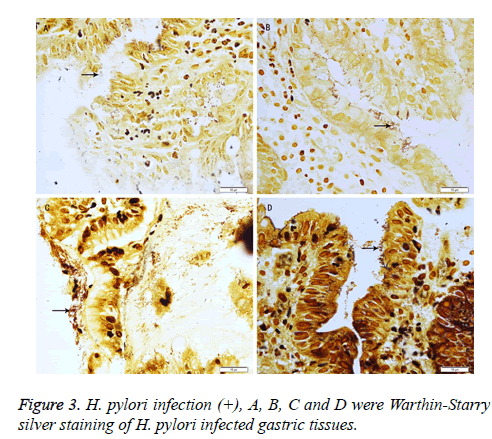
The diagnosis of IM was performed independently by three experienced pathologists according to the diagnostic criteria of Chinese Anti-Cancer Association 1998. Any disagreement was resolved by discussion. H. pylori infection (+) was defined when H. pylori was present in one and/or two slice(s). Statistical analysis was performed using SPSS version 17.0 statistical software (Chicago, IL, USA). Correlation was assessed by the Spearman rho correlation analysis. A value of P<0.05 was considered statistically significant.
Results
Twenty-two male and four female patients aged 41-84 years met the inclusion criteria and were recruited into this study. Nineteen out of twenty-six lesions were from the gastric antrum and five from gastric fundus or body. The main pathologic type of GC was adenocarcinoma, and two patients were diagnosed with gastric stump carcinoma (Table 1).
| Characteristics | No |
|---|---|
| Gender | |
| Male | 22 |
| Female | 4 |
| Age (years) | |
| ≤ 60 | 14 |
| >60 | 12 |
| Pathological types of tumor | |
| Adenocarcinoma | 24 |
| Gastric stump carcinoma | 2 |
| Location of tumor | |
| Fundus and Body | 5 |
| Gastric remnant | 2 |
| Antrum | 19 |
Table 1: Characteristics of enrolled patients.
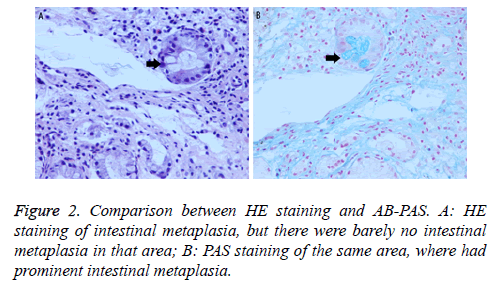
Two gastric tissues (one CAG tissue and one GC tissue) were obtained from each patient, and a total of fifty-two tissues were collected. Correspondingly, there were fifty-two slices for different stainings. By HE staining, thirty-four slices were IM (+), all of which were also IM (+) by AB-PAS. However, nine out of eighteen slices negative for IM by HE staining were IM (+) by AB-PAS. In total, 43 out of 52 slices were IM (+) by AB-PAS (Table 2 and Figure 2).
| Total | Positive | Negative | P | |
|---|---|---|---|---|
| HE | 52 | 34 | 18 | 0.005 |
| AB | 52 | 43 | 9 |
Table 2: IM status detected by different staining.
In addition, 21 out of 26 patients were H. pylori (+). The correlation coefficient between H. pylori infection and IM (AB-PAS) was 0.41 (P<0.05) (Table 3 and Figure 3).
| Total | (+) | (-) | Correlation coefficient | P | |
|---|---|---|---|---|---|
| IM | 26 | 25 | 1 | 0.41 | 0.038 |
| H. pylori | 26 | 21 | 5 |
Table 3: Correlation between IM (AB-PAS) and H. pylori infection.
Discussion
HE staining is the most common used method in the diagnosis of IM. Although the classifications of IM according to HE staining are comprehensive, it is still complex in procedures and evaluation criteria. IM classifications by AB-PAS are concise and explicit (none, existence). In this study, IM (+) slices in HE staining were also IM (+) in AB-PAS, but a half of IM (-) slices in HE staining were IM (+) in AB-PAS, the severity of which were all mild. This means AB-PAS may identify earlier IM, but the specific reasons are unknown. It is possible that goblet cells in the gastric mucosa secret acidic proteins before they become mature. Thus, further studies are needed to confirm the results.
H. pylori are involved in the pathogenesis of GC [9]. Some studies have reported that they contribute to GC by mainly regulating genes (ras, p53, c-myc, etc), cytokines, proliferation and apoptosis of gastric epithelial cells [9,17,18]. GC secondary to IM is also considered as one of consequences due to H. pylori infection, but evidence is still conflicting [16]. In this study, the correlation coefficient between H. pylori infection and IM was 0.41 (P<0.05), implying a correlation between them. There might be several reasons accountable for this correlation. First, the sample size in this study was small, and only 21 out of enrolled patients were H. pylori (+). Second, H. pylori can be classified into two types: CagA positive and CagA negative. CagA positive H. pylori are highly pathogenic while CagA negative H. pylori are not [19]. We did not detect whether H. pylori detected in 21 H. pylori (+) patients were pathogenic H. pylori. Although the correlation coefficient was only 0.41, we still speculate that IM is one of mechanisms underlying the H. pylori induced carcinogenesis according to above factors.
Undoubtedly, it will take a remarkably long time evolving from CAG to GC. In our study, it was about 8 months (data not shown). Possible explanation might be that patients visit hospital only when they have some symptoms such as abdominal distension, abdominal pain, and dyspepsia and they might have suffered from CAG for a long time, or even decades before diagnosis. A majority of subjects died at the time of study and thus we were unable to confirm the exact time when patients got sick. More exactly, the “8 months” were the time interval between the diagnosis of CAG and GC.
In the present study, patients with CAG and GC occurring in the same location were recruited in order to assure that GC evolved from CAG, which reduces the error. Previously, it was difficult for gastroscopists to obtain the gastric mucosa from the same point at different time points. Marking Targeting Biopsy (MTB) is now considered as an accurate assisted biopsy technique during gastroscopy. However, repeated injection of MTB solution is necessary for long-term supervision, and may result in inflammation of the gastric mucosa and finally confuse the histopathological evaluation; despite MTB solution was not harmful. Computer Simulation Making Targeting Biopsy (CSMTB) is a technique which can automatically memorize and identify the original lesion of biopsy without solution injection. Ever since the first use in clinical practice, it has never stopped being improved.
There were still several limitations in this study. First, this study was retrospective, and incomplete patients’ information was inevitable. Second, it was difficult to obtain the gastric tissues due to a long time span. More studies are still required before a more preferable method for the diagnosis of IM is developed.
Conclusions
Taken together, AB-PAS is superior to HE staining in the diagnosis of IM, but its clinical application is needed to be further confirmed in future prospective studies. The correlation coefficient between H. pylori infection and IM was 0.41, implying an association between them. Thus, IM may be one of mechanisms underlying the H. pylori induced carcinogenesis.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (81372623), Zhejiang Province Key Science and Technology Innovation Team (2013TD13), Zhejiang Provincial Medical and Health Research Plan (201476310).
References
- Yan S, Li B, Bai ZZ, Wu JQ, Xie DW, Ma YC, Ma XX, Zhao JH, Guo XJ. Clinical epidemiology of gastric cancer in Hehuang valley of China: a 10-year epidemiological study of gastric cancer. World J Gastroenterol 2014; 20: 10486-10494.
- Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet 1975; 2: 58-60.
- Goldenring JR. Gastric intestinal metaplasia and tamoxifen: can we reverse the inevitable? Dig Dis Sci 2014; 59: 1078-1079.
- Zhou L, Lin S, Ding S, Huang X, Jin Z, Cui R, Meng L, Li Y, Zhang L, Guo C, Xue Y, Yan X, Zhang J. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chin Med J (Engl) 2014; 127: 1454-1458.
- German SV, Zykova IE, Modestova AV, Ermakov NV. Epidemiological characteristics of Helicobacter pylori infection in Moscow. Gig Sanit 2011; 44-48.
- Eshraghian A. Epidemiology of Helicobacter pylori infection among the healthy population in Iran and countries of the Eastern Mediterranean Region: a systematic review of prevalence and risk factors. World J Gastroenterol 2014; 20: 17618-17625.
- Hsu WY, Lin CH, Lin CC, Sung FC, Hsu CP. The relationship between Helicobacter pylori and cancer risk. Eur J Intern Med 2014; 25: 235-240.
- Bornschein J, Malfertheiner P. Helicobacter pylori and gastric cancer. Dig Dis 2014; 32: 249-264.
- Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Helicobacter pylori infection, glandular atrophy and intestinal metaplasia in superficial gastritis, gastric erosion, erosive gastritis, gastric ulcer and early gastric cancer. World J Gastroenterol 2005; 11: 791-796.
- Lukes P, Pavlik E, Potuznikova B, Nartova E, Foltynova E, Plzak J, Katra R, Sterzl I, Bartunkova J, Betka J, Astl J. Detection of Helicobacter pylori in oropharyngeal lymphatic tissue with real-time PCR and assessment of its carcinogenic potential. Eur Arch Otorhinolaryngol 2014; 271: 399-405.
- Liu HF, Liu WW, Wang GA, Teng XC. Effect of Helicobacter pylori infection on Bax protein expression in patients with gastric precancerous lesions. World J Gastroenterol 2005; 11: 5899-5901.
- Ricci V, Ciacci C, Zarrilli R, Sommi P, Tummuru MK, Del Vecchio Blanco C, Bruni CB, Cover TL, Blaser MJ, Romano M. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect Immun 1996; 64: 2829-2833.
- Lan J, Xiong YY, Lin YX, Wang BC, Gong LL, Xu HS, Guo GS. Helicobacter pylori infection generated gastric cancer through p53-Rb tumor-suppressor system mutation and telomerase reactivation. World J Gastroenterol 2003; 9: 54-58.
- Yoon JH, Choi WS, Kim O, Park WS. The role of gastrokine 1 in gastric cancer. J Gastric Cancer 2014; 14: 147-155.
- Mansour-Ghanaei F, Joukar F, Mojtahedi K, Sokhanvar H, Askari K, Shafaeizadeh A. Does treatment of Helicobacter pylori infection reduce gastric precancerous lesions? Asian Pac J Cancer Prev 2015; 16: 1571-1574.
- Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev 2015; 20: 25-40.
- Suganuma M, Yamaguchi K, Ono Y, Matsumoto H, Hayashi T, Ogawa T, Imai K, Kuzuhara T, Nishizono A, Fujiki H. TNF-alpha-inducing protein, a carcinogenic factor secreted from H. pylori, enters gastric cancer cells. Int J Cancer 2008; 123: 117-122.
- Xiong H, Du W, Sun TT, Lin YW, Wang JL, Hong J, Fang JY. A positive feedback loop between STAT3 and cyclooxygenase-2 gene may contribute to Helicobacter pylori-associated human gastric tumorigenesis. Int J Cancer 2014; 134: 2030-2040.
- Erdogdu C, Saribas Z, Akyon Yilmaz Y. Detection of cagA and vacA genotypes of Helicobacter pylori isolates from a university hospital in Ankara region, Turkey. Turk J Med Sci 2014; 44: 126-132.