ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 12
A study on performance and safety test of infusion pump devices
1Department of Biomedical Engineering, Standard Research Institute, Karaj, Iran
2Department of Medical Physics, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
3Department of Electrical Engineering, Iranian Research Organization for Science and Technology (IROST), Tehran, Iran
4Department of Biomechanics, Science and Research Branch, Islamic Azad University, Tehran, Iran
- *Corresponding Author:
- Tavakoli Golpaygani A
Department of Biomedical Engineering
Standard Research Institute, Iran
Accepted date: July 13, 2016
Nowadays, more than 10,000 different types of medical devices can be found in hospitals. About 80% of the patients receive intravenous (IV) therapy. Infusion devices are mainly used in clinical applications and patients’ homes to provide critical care, perioperative care and pain management. The fluids’ infusion uses a complex of designs which lead to the feed ability, hydrate, medicate or blood loss replacement and more rapid IV administration because it is pumped directly into the blood. The patients are normally in an intensive care situation and their life is directly dependent on the performance of a device. On the other hand, they are maybe unconscious, have impaired reactions, or have been made insensitive to pain. Therefore, they are exposed to hazards due to lack of information. During last five years, the FDA has received many reports of adverse events regarding the use of infusion pumps. The reports have stated serious injuries and hundreds of deaths. So, the reliability of infusion pumps is a matter of importance and it must be regularly the performance and safety of these devices verified at least annually by using adequate analyzer via expert persons.
Keywords
Reliability, Infusion pump, Patient safety, Medical electrical equipment.
Introduction
Nowadays, more than 10,000 different types of medical devices can be found in hospitals. About 80% of the patients receive intravenous (IV) therapy. Infusion devices are mainly used in clinical applications and patients’ homes to provide critical care, perioperative care and pain management. The fluids’ infusion uses a complex of designs which lead to the feed ability, hydrate, medicate or blood loss replacement and more rapid IV administration because it is pumped directly into the blood. The patients are normally in an intensive care situation and their life is directly dependent on the performance of a device. On the other hand, they are maybe unconscious, have impaired reactions, or have been made insensitive to pain. Therefore, they are exposed to hazards due to lack of information. During last five years, the FDA has received many reports of adverse events regarding the use of infusion pumps. The reports have stated serious injuries and hundreds of deaths. So, the reliability of infusion pumps is a matter of importance and it must be regularly the performance and safety of these devices verified at least annually by using adequate analyzer via expert persons, [1-4].
Technical background
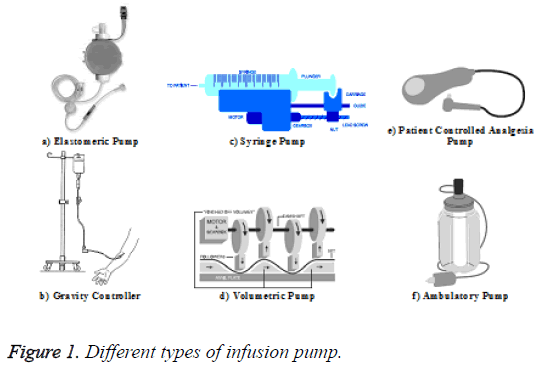
Infusion pumps are used to pump the fluids, medications, and other minerals into the patient body for different purposes. An infusion circuit contains some items including the fluid container and a length of flexible tubing which connect to the patient’s catheter. Generally, the infusion pumps are used when the chosen solution shall be delivered by much accuracy or need for speed. Infusion pump models vary in design and capabilities. Differences can exist, for example, in the application purposes, pumping mechanism employed, and the size of the unit. The type of pump, which is used, depends on the patient’s requirements such as the required volume and desired infusion speed. The pattern of fluid delivery is dependent on the type of pump used. Figure 1 shows the different pump types [5,6].
Materials and Methods
The aim of performing test is to verify whether the device pumps the required flow rate, volume, and bolus with required rate of accuracy; occlusion alarms are activated during emergency conditions, and the device is safe for patient and operator use? It is an important point that the testing conditions mimic the real-life settings. They show what the manufacturer suggests to make sure that the equipment performance is based on its required specification. In this study fifty infusion pump, four (4) different brands were used at ten (10) hospitals were tested. The setup that was used for the accuracy of controls and instruments measurements was designed in accordance with the particular safety standard IEC 60601-2-24. Flow rates were measured with a Neteck Biomedical Analyzer (IPA 2000) and a Fluke Electrical Safety Analyzer (ESA 620) used for general electrical safety evaluations for measuring the patient leakage currents.
There are several parameters that impact the performance of infusion pump devices. After performing a visual check, the performance of the system is assessed and electrical safety test should also be performed to insure that all patient safety points and instrument reliability are met according to the test procedure. According to the particular standard IEC 60601-2-24, flow rate accuracy of delivered fluid, occlusion pressure, bolus and total volume measurement should be performed in different rates and different output environment conditions. Also, several of the electrical safety tests according to general standard for medical electrical equipment (IEC 60601-1), especially patient leakage currents were performed in all infusion pumps [7,8].
Results and Discussion
The performance and safety testing of infusion pump devices in use at ten clinical centers was evaluated. Table 1 shows a brief history of achieved results as a sample for ten units.
| Items | U1 | U2 | U3 | U4 | U5 | U6 | U7 | U8 | U9 | U10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy of Flow Rate | * | * | *** | ** | ** | * | *** | * | * | ** |
| Accuracy of Total Infused Volume | * | * | ** | * | ** | * | ** | * | * | ** |
| Occlusion Pressure | √ | √ | × | √ | × | √ | × | √ | √ | × |
| Bolus | √ | √ | × | √ | √ | √ | × | √ | √ | × |
| Batteries | AC | AC | RE | RE | RE | AC | RE | AC | AC | RE |
| Using Standard Syringe or Tubing Set | √ | √ | × | √ | × | × | × | √ | √ | × |
| Patient Leakage Current | √ | √ | × | √ | × | √ | √ | √ | √ | √ |
| Percentage Error Based on Set Point | ||||||||||
| *5-10% | ** 10-20% | *** Above 20% | ||||||||
| √ -In Standard Limitation | × - Out of Standard Limitation | |||||||||
| AC: Accept | RE: Reject | |||||||||
| Electrical Safety | ||||||||||
| Patient Leakage Current | Patient Leakage Current | |||||||||
| Normal Condition | Fault Condition | |||||||||
| CF Type: 10 µA , Bf Type: 100 µA | CF Type: 50 µA , BF Type: 500 µA | |||||||||
Table 1: A brief summary of evaluated technical result for ten units.
Quantitative analysis of flow rate accuracy measurements, showed the amount of the obtained results in many units are critical and have less value over the standard limitations, especially in devices with inappropriate IV set. It has been seen, the usage of a wrong or nonstandard syringe or tubing set, which is not made based on the approved technical specifications, increases the occurred error percent and the inaccuracy 10 to 20%. The position of drip detector is an important factor in infusion pumps. If the detector is installed near the injection location, then the error percent can increase up to 25%. The reduction of battery power is one of the effective factors on the performance of the infusion pumps. It has been seen that the low quality batteries could reduce the outflow rate between 10 to 30%. This could be occurring on the condition that the battery power reaches to 25% under the full power limit.
General electrical safety evaluations for measuring the patient leakage currents carried out for all of the under test devices. In some cases the amount of Patient leakage currents were over the standard limitations.
Conclusion
Due to design and manufacturing technology development of the pumps, which enables the programmers to program different injection patterns in new pumps, it is required to consider supplementary professional training courses for the users to enable them to use all available pump options and reduce the human errors. Also, Acquiring results indicate a need for new and severe regulations on periodic performance verifications and medical equipment quality control program especially in high risk instruments.
References
- Tavakoli Golpaygani A, Movahedi MM, Reza M. A Study on Performance and Safety Tests of Electrosurgical Equipment. J Biomed Physics Eng 2016.
- Davis W. Infusion Devices Training Tutorial 2010.
- Keay S, Challander C. The Safe Use of Infusion Devices. Anaesth Crit Care Pain 2004; 4: 81-85.
- U.S Food and Drug Administration. FDA Launches Initiative to Reduce Infusion Pump Risks 2010.
- Khandpour RS. Handbook of Biomedical Instrumentation. Mc Graw Hill, New York 2014.
- Street L. Introduction to Biomedical Engineering technology. CRC Press, USA, 2008.
- IEC 60601-1-1, Medical Electrical Equipment- Part1: General requirements for Safety1: Collateral Standard: Safety Requirements for Medical electrical Systems.
- IEC 60601-2-24, Medical electrical equipment - Part 2-4: Particular requirements for the safety of infusion pumps and controllers.