ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 3
A study of inflammatory markers and their correlation with severity, in patients with chronic heart failure
Department of Medicine, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, India
1Interdisciplinary Biotechnology Unit, Aligarh Muslim University, Aligarh, India.
- *Corresponding Author:
- Nooralam Ansari
F-1, Azim Estate Sir Syed Nagar
Aligarh (Uttar Pradesh)-202002 India
Accepted June 07 2012
Immune system activation in CHF has received considerable interest in the last decade. It is now becoming apparent that inflammatory mediators play a crucial role in the development of CHF. Therefore, the present study was undertaken with the aim to evaluate the association between inflammatory markers and CHF and to correlate them with severity of CHF. A total of 42 subjects were initially classified into 2 groups. First group was control group. Second group was CHF group, which on the basis of their current NYHA status was further subdivided into 2 groups, as either having Mild CHF (including patients in NYHA class I or II, n=13) or Severe CHF (including patients in NYHA class III or IV, n=19). Assessment of physical parameters included heart rate and BMI. Plasma levels of hs-CRP and TNF-α were estimated. Routine blood investigations included hemoglobin, plasma glucose, blood urea, serum creatinine, serum lipids, serum sodium and potassium. Echocardiography was done on every patient. BMI in severe CHF group was significantly lower when compared with control (p<0.01) and mild CHF group (p<0.05). Heart rate was also significantly raised in CHF group. LVEF and LVFS was significantly lower in patients with mild CHF (p<0.01 for both) and severe CHF (p<0.01for both) when compared with control. Mean hemoglobin was significantly lower in patients of mild CHF (p<0.05) and severe CHF (p<0.05) when compared with controls. Circulating level of hs-CRP and TNF-α was significantly elevated in CHF patients (p<0.01), and increased with severity of CHF (p<0.01). There was a strong correlation of NYHA functional class with hs-CRP (r=0.527, p<0.01) and TNF-α (r=0.89, p<0.01). hs-CRP and TNF-α levels are significantly elevated in patients with CHF. There is a strong association between hs-CRP & TNF-α levels and severity of chronic heart failure. BMI is a significant associated with levels of hs-CRP and TNF-α. No correlation was found between LVEF and levels of hs-CRP and TNF-α. TNF-α and hs-CRP levels are elevated irrespective of the presence or absence of coronary artery disease suggesting, CHF as a final common pathway for a variety of cardiac disorders.
Keywords
CHF (Chronic heart failure), NYHA (New York Heart Association), hs-CRP (High sensitivity C-reactive protein), TNF-α (Tumor necrosis factor-α), Cytokines, BMI (body mass index).
Introduction
Chronic heart failure (CHF) is a complex syndrome, which is characterized by shortness of breath, fatigue, congestion, and cachexia, and symptoms related to inadequate tissue perfusion, fluid retention, and neurohormonal activation.
Despite significant improvement in medical therapy of cardiovascular diseases, CHF remains a serious clinical problem. It represents a major public health burden with high morbidity and mortality. Virtually any cardiac disease can ultimately lead to heart failure, though the initial event leading to the development of this syndrome in many cases remains unknown.
It has been suggested by various investigators that CHF should be seen as a neurohormonal model, in which the syndrome of CHF progresses because of activation of neurohormones and proinflammatory cytokines following an initial cardiac insult or injury or a mutation of the genetic programme [1], overexpression of these biologically active molecules exerts toxic effects on the heart and circulation[ 2]. The role of immune system activation in CHF has received considerable interest in the last decade. It is now becoming increasingly apparent that inflammatory mediators play a crucial role in the development of CHF[3], and several strategies to counterbalance various aspects of the inflammatory response are considered. One of the possible targets include pro- and anti-inflammatory cytokines and their receptors. The concept that biological markers may accurately predict the outcome of CHF patients is an attractive one. Several reports have indicated that elevated blood levels of inflammatory markers are associated with an adverse prognosis in CHF patients[4, 5].
Therefore, the present study was undertaken with the aim to evaluate the association between inflammatory markers and CHF and to correlate them with severity of CHF according to New York Heart Association (NYHA) functional class[6].
Materials and Methods
The study was conducted from January 2006 to June 2007 in the department of Medicine, J.N. Medical College and Hospital, AMU, Aligarh. A total of 42 subjects including 32 patients (attending medicine OPD, IPD, CCU and Cardiology Clinic) having chronic heart failure, diagnosed according to the Framingham criteria for heart failure[ 7] and 10 healthy age and gender matched controlled subjects with no apparent cardiovascular disease, were recruited in this study after taking informed consent from them.
Patients having renal failure, chronic liver disease, myocardial infarction within the previous three months, diabetes mellitus, malignancy, acute or chronic inflammatory or infectious diseases such as infection or sepsis, rheumatoid arthritis, connective tissue diseases and other autoimmune diseases known to cause elevation in High sensitivity C-reactive protein (hs-CRP) and Tumor necrosis factor-α (TNF-α) levels, were excluded from this study.
Study design
The study was a case control cross-sectional prospective study. The study protocol was approved by the Board of Studies of the Department of Medicine, JNMC in November 2005. A detailed history, physical and systemic examination including measurement of height, weight, heart rate, blood pressure and body mass index (BMI) was carried out in every subject who entered the study as per a pre-designed proforma for assessing the signs of chronic heart failure and also the presence of any exclusion criteria as previously discussed.
The 42 subjects included in this study were initially classified into two groups. First group was control group, it included ten healthy age and gender matched control subjects with no apparent cardiovascular disease. Second group was CHF group; it included 32 patients having CHF. Now in CHF group, depending upon the severity of symptoms, clinical status of all patients was defined using the NYHA classification. The patients of CHF group, on the basis of their current NYHA functional status were further subdivided into 2 groups, as either having “Mild CHF” (including patients in NYHA class I or II, n=13) or “Severe CHF” (including patients in NYHA class III or IV, n=19).
2D/M-mode and Color Doppler Echocardiography were done on every patient for left ventricular ejection fraction (LVEF) and left ventricular functional shortening (LVFS), using ATL (A Philips medical system company)- HDI 1500 echocardiography machine.
Blood samples were taken for the estimation of hemoglobin, plasma glucose, blood urea, serum creatinine, serum sodium, serum potassium, serum lipid profile and for the plasma levels of inflammatory cytokine TNF-α and hs- CRP. The hs-CRP estimation was performed by using UBI MAGIWEL CRP-quantitative AD-401 kit, a solid phase enzyme linked immunosorbant assay (ELISA) as per instructions of the manufacturer (supplied with kit) with reference range for CRP being 0.0-0.8 mg/dl. TNF-α was estimated by antigen capture ELISA. In the procedure, test sera from different patient were collected and serial dilution were made in appropriate coating buffer starting from 1:100 and 100μl from each dilution were plate in 96 well polystyrene microtitre plates and kept at 4ºC for overnight incubation. After proper washing of the wells, the unoccupied sites were blocked for 1 hour at room temperature with 200μl of PBS (pH 7.0) containing 2% BSA per well. After that the plate was washed five times with PBS-0.05 % Tween 20. Anti TNF-α sera raised in rabbit was diluted upto 1:1000 in PBS-1% BSA and 100μl was plated into each well. The plate was kept at 37ºC for 1 hour. After usual washing, all the wells were charged with 100μl HRPO (Horseradish peroxidase) conjugated rabbit anti mouse IgG (total antibody) and kept for 1 hr at 37ºC. After washing with PBS-T the plate was incubated with 100μl OPD (Ortho phenyle diamine) substrate solution for 5-10 min and color production was stopped by adding stopping solution (2N H2SO4). All assays were performed in triplicate. Absorbance was read at 492 nm using ELISA reader. The concentration of TNF-α in the sera sample was calculated for each patient using a linear-regression equation obtained from the standards curve prepared with rTNF-α.
Statistical analysis
All statistical data were analyzed by using SPSS software version 10 Statistical package for windows (Chicago. Inc.). Continuous variables were expressed as mean ± standard deviation (Gaussian distribution) or range and qualitative data was expressed as percentage. Depending on normality distribution, unpaired t test for independent samples was used for comparing continuous variables between two groups. ANOVA or analysis of variance with Scheffe’s post-hoc analysis was used for comparing data between groups. Linear relationship between variables was analyzed using Pearson’s correlation coefficient and significance of ‘r’ was tested. A Stepwise multivariate regression analysis was used to study the determinants of TNF-a and CRP. All p values were two tailed and values of p<0.05 was considered statistically significant. All confidence intervals were calculated at 95% level.
Results
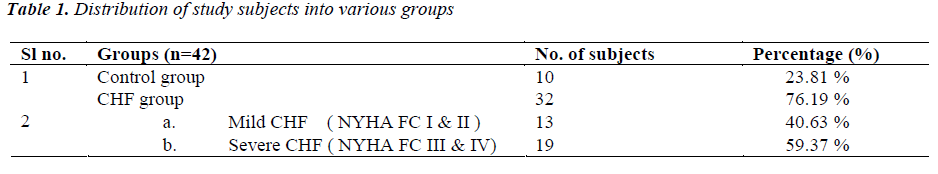
(Tables 1,2) Subjects were classified into two groups as CHF group (n=32, 76.19%) and control group (n=10, 23.81%). CHF group, was further subdivided into two groups as either having mild CHF, n=13, 40.63% (including patients in NYHA class I or II) or severe CHF, n=19, 59.37% (including patients in NYHA class III or IV).
In control group there were 7 (70%) males and 3 (30%) females. In mild CHF group there were 09 (69.23 %) males and 04 (30.77 %) females and in severe CHF group there were 13 (68.42 %) males and 06 (31.58 %) females. Mean age of subjects in control group was 53.30 ± 9.0 years (Range 39-66 years). In mild CHF group, mean age was 52.77 ± 9.9 years and in severe CHF group, it was 57.74 ± 9.22 years.
Etiology of heart failure was Ischaemic heart disease (IHD) in 18 (56.25%), Valvular heart disease (VHD) in 07 (21.87%), Dilated cardiomyopathy (DCM) in 04 (12.50%) and hypertensive heart disease (HHD) in 03 (9.30%) patients. 12 (37.5 %) patients had NYHA class IV symptoms on presentation, 07 (21.87%) had class III symptoms; 08 (25%) had class II and 05 (15.63%) had class I symptoms.
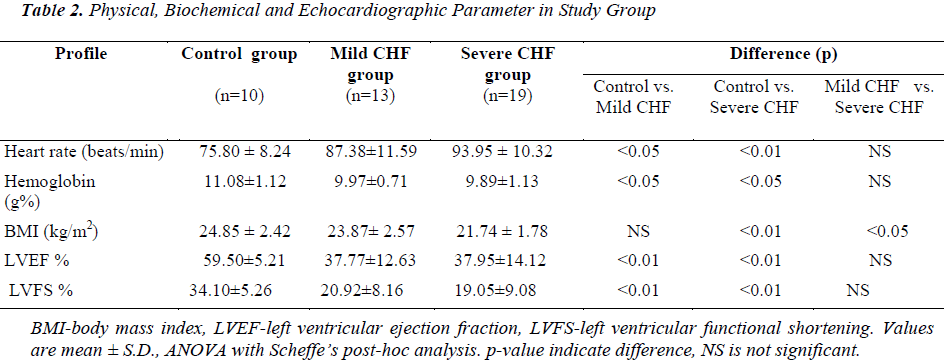
Mean BMI in control group was 24.85 ± 2.42 kg/m2. Mean BMI in mild CHF and severe CHF group was 23.87 ± 2.57 and 21.74 ± 1.78 kg/m2, respectively. BMI in severe CHF group was significantly lower when compared with control (p<0.01) and mild CHF group (p<0.05), but there was no significant difference in BMI between control and mild CHF group (p=Not Significant).
In the control group mean left ventricular ejection fraction (LVEF) and mean left ventricular fractional shortening (LVFS) was 59.50 ± 5.21 % (range 50-67%) and 34.10 ± 5.26% respectively. In mild CHF group mean LVEF and LVFS was 37.77 ± 12.63% (range 15-55%) and 20.92 ± 8.16%, respectively. In severe CHF group mean LVEF and LVFS was 37.95 ± 14.12% (range 15-56 %) and 19.05 ± 9.08%, respectively. When compared with controls LVEF and LVFS was significantly lower in patients with mild CHF (p<0.01) and severe CHF (p<0.01), but there was no significant difference in LVEF between mild CHF and severe CHF group (p=NS).
Mean hemoglobin in the control group was 11.08 ± 1.12 gm%, while in mild CHF and severe CHF group it was 9.97 ± 0.71 and 9.89 ± 1.13 gm%, respectively. When compared with controls mean hemoglobin was significantly lower in patients of mild CHF (p<0.05) and severe CHF (p<0.05), but there was no significant difference in mean hemoglobin between mild CHF and severe CHF group (p>0.05). There was no significant difference in mean serum sodium, mean serum creatinine, total cholesterol, HDL, LDL and triglyceride between CHF and control groups.
Immunological Parameters
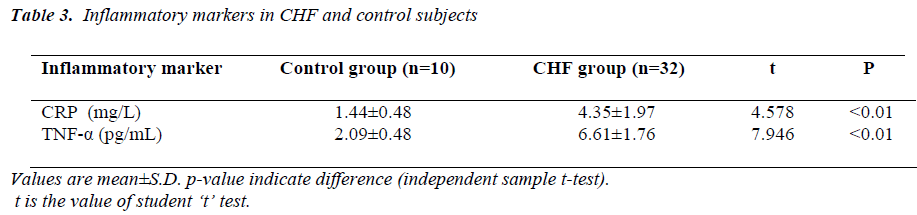
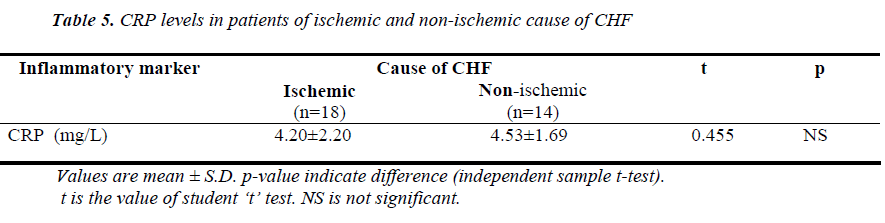
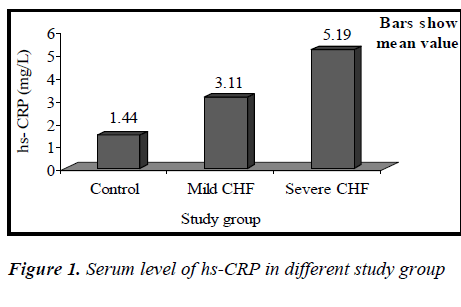
(Tables 3, 4, 5 and Figures 1 & 2) Mean value of CRP in the control group was 1.44 ± 0.48 mg/L (range 0.76-2.10mg/L), while in CHF group (mild and severe CHF combined) it was 4.35 ± 1.97 mg/L (range 1.28-8.62 mg/L). In CHF group, 21 (65.63%) had CRP value > 3 mg/L and 11 (34.37%) had CRP value between 1-3 mg/L. None had CRP value < 1 mg/L.
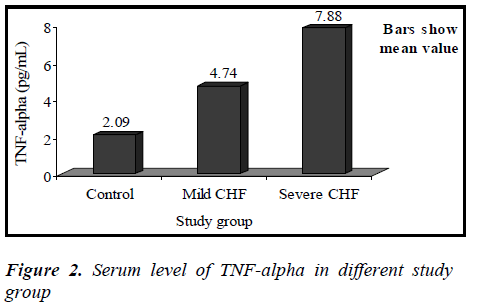
Mean value of TNF-α in the control group was 2.09 ± 0.48 pg/mL (range 1.53-2.86 pg/mL), while in CHF group (mild and severe CHF combined) it was 6.61 ± 1.76 pg/mL (range 3.70-9.12 pg/mL).
When compared with controls by independent samples ttest, mean value of CRP was significantly higher in patients of CHF (t=4.578, p<0.01), similarly mean value of TNF-α was also significantly higher in CHF group when compared with controls (t= 7.946, p<0.01).
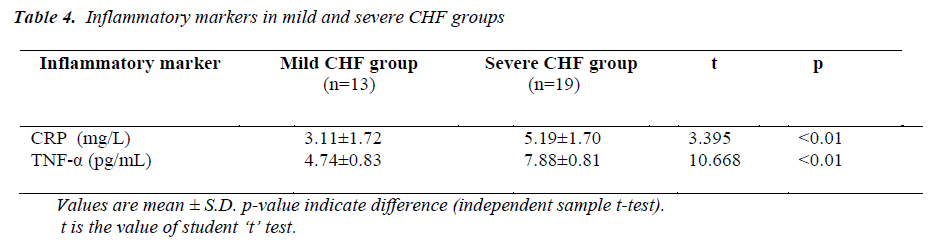
Mean value of CRP in the mild CHF group was 3.11± 1.72 mg/L (range 1.28-6.79mg/L), while in severe CHF group it was 5.19±1.70 mg/L (range 2.82±8.62mg/L).
Mean value of TNF-α in the mild CHF group was 4.74 ± 0.83 pg/mL (range 3.70-6.13 pg/mL), while in severe CHF group it was 7.88 ± 0.81 pg/mL (range 6.19-9.12 pg/mL).
Between mild CHF and severe CHF group, mean value of CRP was significantly higher in severe CHF group (t=3.395, p<0.01), similarly mean value of TNF-α was also significantly higher in severe CHF group (t= 10.668, p<0.01).
On the basis of etiology, patients of CHF were again divided into ischemic group (n=18, 56.25%) and nonischemic group (n=14, 43.75%) and the levels of CRP was compared between the two groups. There was no significant difference in the mean value of CRP between the two groups (p>0.05).
Though females in the study had slightly higher mean value of CRP (4.87±2.10mg/L) and TNF-α (6.96±1.98 pg/mL) then the males (CRP=4.02±1.91mg/L, TNF-α= 6.47±1.72pg/mL), but the difference was not statistically significant (p>0.05).
Pearson’s correlation of TNF-α with other variables
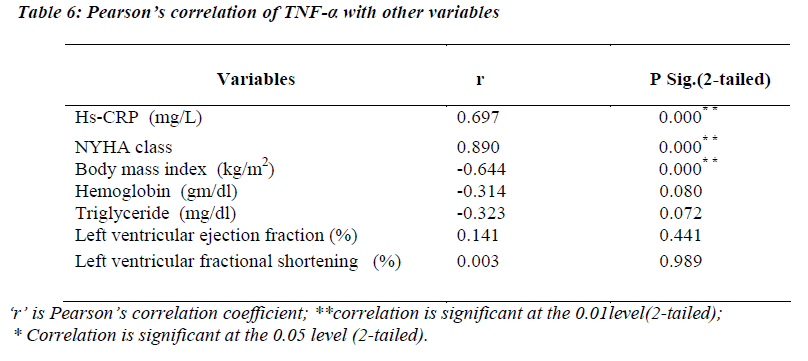
(Table 6) TNF-α levels were significantly correlating with hs-CRP levels (r=0.697; p<0.01) and NYHA functional class (r=0.890; p=<0.01). A strong negative correlation was found between TNF-α and body mass index (r= -0.644; p<0.01).
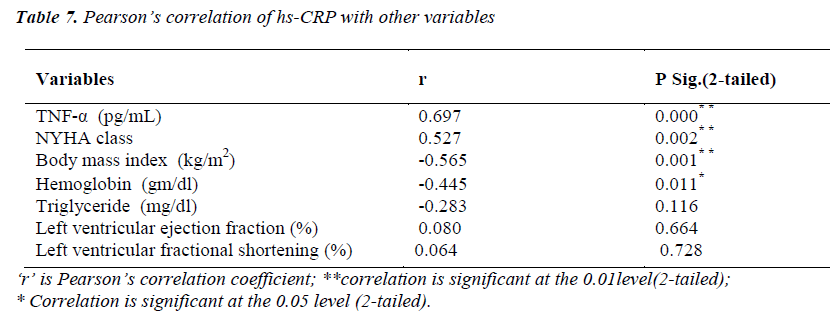
Pearson’s correlation of hs-CRP with other variables
(Table 7) hs-CRP levels were significantly correlating with TNF-α level (r=0.697; p<0.01) and NYHA functional class (r=0.527; p<0.01). A significant negative correlation of hs-CRP was found with body mass index (r= -0.565; p<0.01) and hemoglobin (r= -0.445; p<0.05).
Discussion
A total of 42 subjects including 32 patients having chronic heart failure and 10 healthy age and gender matched controlled subjects with no apparent cardiovascular disease, were recruited in this study. The mean age of subjects in control and CHF group was 53.30 ± 9.0 and 55.72 ± 9.66 years respectively. The majority of the subjects in the study group were above 45 years of age. This is in accordance with the fact that, there is a progressive increase in the incidence and prevalence of heart failure with age [8].
We also observed that the patients with CHF had faster heart rate and lower body mass index. BMI in severe CHF group was significantly lower when compared with control (p<0.01) and mild CHF group (p<0.05). This indicates that both cardiovascular and metabolic reserves deteriorate as heart failure progresses. The concept and mechanisms of heart failure evolved from a cardiorenal and cardiocirculatory dysfunction to neurohumoral disorder. The syndrome of cardiac cachexia is characterized by a severe catabolic/anabolic imbalance in favor of catabolic metabolism. In cachectic CHF patients, catabolic factors that act to increase protein and fat tissue degradation and stimulate energy production are increased from the effects of catecholamines, cortisol, and TNF-α [9,10,11]. Similar results were obtained from study by Ju-Pin Pan et al., who observed that, patients with severe CHF had smaller BMI along with faster HR rate[12].
When compared with controls LVEF and LVFS was significantly lower in patients with mild CHF (p<0.01 for both) and severe CHF (p<0.01for both). Though left ventricular ejection fraction and left ventricular fractional shortening were significantly, lower in patients with heart failure in our study, we were not able to find any correlation between ejection fraction and circulating levels of inflammatory markers( hs-CRP and TNF-α). Interestingly, previous studies have shown conflicting results. In some prior studies of patients with systolic heart failure, left ventricular ejection fraction has not correlated well with levels of inflammatory markers[13,14]. On the contrary, some researchers have found inverse correlation between LVEF and hs-CRP[15].
Our study showed that the circulating level of hs-CRP was significantly elevated in patients with chronic heart failure (p<0.01), and that this increase parallels the severity of CHF (p<0.01), showing a strong correlation between hs-CRP and NYHA functional class(r= 0.527,p<0.01). It was also observed that the elevation in the levels of CRP was independent of the sex of patient, explaining that the increase of hs-CRP levels in CHF seemed to be a general phenomenon unrelated to the sex of the patient. This is consistent with the recent view that chronic heart failure may in part, be an inflammatory disease. Yin WH. et al., on a series of 108 patients with LVEF <50%, observed that hs-CRP levels were significantly increased with the severity of CHF and were correlating with NYHA functional class and that higher levels were associated with an increased risk of death or hospitalization [14]. In this study, it was also observed that increase in hs-CRP levels in patients with CHF was unrelated to the presence or absence of coronary artery disease, which suggests that CHF is the final common pathway of a variety of cardiac disorders. This finding is consistent with previous study by Yin W.H. and colleagues [14].
Our study also highlighted the significant correlation of hs-CRP with circulating levels of TNF-α and a negative correlation with body mass index (BMI) and hemoglobin level. In multivariate regression analysis, NYHA functional class (t=2.202,p<0.05) and BMI (t=2.068,p<0.05) came out to be significant predictor of variance of the CRP level.
Mean hemoglobin was significantly lower in patients of mild CHF (p<0.05) and severe CHF (p<0.05) when compared with controls. Anemia is common in patients with heart failure, associated with worse symptoms, and increased mortality [16]. In one study patients with anemia had higher hs-CRP levels, and there was an inverse relationship between changes in hs-CRP and hemoglobin [17]. It is likely that the anemia of chronic heart failure is multi- factorial. Activation of cytokines such as TNF-α, IL-6, and IL-1B can alter iron homeostasis, reduce the production of erythroid progenitor cells and erythropoietin, and shorten the life span of red cells, leading to anemia [ 18].
The complement activation by CRP in CHF is supposed to be an important contributor to the production of TNF- α [19]. In some inflammatory models, the presence of complement activation is required for TNF-α-mediated diseases[20,21]. Our data showed that there is a correlation between circulating levels of hs-CRP and TNF-α, which is consistent with these findings.
Our study showed that the circulating level of TNF-α was significantly elevated in patients with chronic heart failure (p<0.01), and increased with the severity of CHF (p<0.01) and there is a strong correlation between TNF-α and NYHA functional class(r=0.890, p<0.01). Elevated TNF-α levels have been demonstrated in patients with heart failure, particularly associated with an increased severity of heart failure[22,23].
Sharma, Bolger, Li, Davlouros, Volk, Poole-Wilson et al in their study [24] also found that plasma TNF-α levels were significantly higher in patients with moderate-tosevere symptoms (NYHA FC III/IV) compared with patients who were asymptomatic or had mild symptoms (NYHA FC I/II).
In this study, we found significant inverse correlation between TNF-α and body mass index (r= -0.644, p<0.01). In multivariate regression analysis, NYHA functional class, hs-CRP and BMI came out to be significant predictor of TNF-α level. TNF-α over-expression causes skeletal myopathy and endothelial dysfunction and catabolism in CHF patients through free radical and nitric oxide over production [25], which is associated with impairment of exercise capacity and cachexia [26].
Conclusion
In conclusion, we extend previous reports that the circulating levels of inflammatory markers, hs-CRP and TNF- α are significantly elevated in patients with chronic heart failure, supporting the view, that chronic heart failure is in part, an inflammatory disorder. There is a strong association between hs-CRP and TNF-α levels and the severity of chronic heart failure according to NYHA class. There is a correlation between circulating levels of hs-CRP and TNF-α, suggesting an inflammatory cytokine cascade in chronic heart failure. Left ventricular ejection fraction by echocardiography do not correlate with levels of hs-CRP and TNF-α. In chronic heart failure, TNF-α and hs-CRP are elevated irrespective of the presence or absence of coronary artery disease suggesting, CHF as a final common pathway for a variety of cardiac disorders. Further studies are required to determine optimal clinical roles of hs-CRP and TNF-α measurement in chronic heart failure.
References
- Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 2002; 91: 988-998.
- Tan LB, Jalil JE, Pick R, Janicki JS, Weber KT. Car- diac myocyte necrosis induced by angiotensin II. Circ Res 1991; 69: 1185-1195.
- Mann DL, Young JB. Basic mechanisms in congestive heart failure: Recognizing the role of proinflammatory cytokines. Chest 1994; 105: 897-904.
- Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D et al. Interleukin-6 spillover in the periph- eral circulation increases with the severity of heart fail- ure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with conges- tive heart failure. J Am Coll Cardiol 1998; 31: 391-398.
- Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C et al. Plasma cytokine parame- ters and mortality in patients with chronic heart failure. Circulation 2000; 102: 3060-3067.
- The criteria committee of the New York Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed. Boston, Mass: Lit- tle, Brown &Co. 1994; 253-256.
- Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemi- ology of heart failure: the Framingham Study. J Am Coll Cardio 1993; 22 (Suppl A): 6A-13A.
- Kannel WB. Epidemiology and prevention of cardiac failure: Framingham Study insights. Eur Heart J 1987; 8: 23-28.
- Poehlman ET, Danforth E. Endurance training in- creases metabolic rate and norepinephrine appearance rate in older individuals. Am J Physiol 1991; 261: 233-A23n9a.nd IS, Ferrari R, Kalra GS, Wahi PL, Poole-Wilson PA, Harris PC. Edema of cardiac origin: studies on body water and sodium, renal function, hemodynamic indexes, and plasma hormones in untreated congestive cardiac failure. Circulation 1989; 80: 299-305.
- Stefan DA, Tuan PC, Piotr P, Derrek H, Jon WS, Wolfgang JK et al. Hormonal changes and cata- bolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation 1997; 96: 526-534.
- Ju-Pin Pan, Tu-Ying Liu, Shu-Chiung Chiang, Yung- Kuo Lin, Chia-Yea Chou, Wan-Leong Chan et al. The Value of Plasma Levels of Tumor necrosis factor-α and interleukin-6 in predicting the severity and prognosis in patients with congestive heart failure. J Chin Med As- soc 2004; 67: 222-228.
- Berton G, Cordiano R, Palmieri R, Pianca S, Pagliara V, Palatini P. C-reactive protein in acute myocardial in- farction: association with heart failure. Am Heart J 2003; 145: 1094-1101.
- Yin WH, Chen JW, Jen HL, Chiang MC, Huang WP, Feng AN et al. Independent prognostic value of eleva- ted high-sensitivity C-reactive protein in chronic heart failure. Am Heart J 2004; 147: 931-938.
- Arroyo-Espliguero R, Avanzas P, Quiles J, Kaski JC. C-reactive protein predicts functional status and correlates with left ventricular ejection fraction in patients with chronic stable angina. Atherosclerosis 2009; 205: 319-324.
- Horowich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anaemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002; 39: 1780-1786.
- Windram JD, Loh PH, Rigby AS, Hanning I, Clark AL, Cleland JGF. Relationship of High-Sensitivity C- Reactive Protein to Prognosis and Other Prognostic Markers in Outpatients With Heart Failure. Am Heart J 2007; 153: 1048-1055.
- Weiss G, Goodnough LT. Medical progress: anaemia of chronic disease. N Engl J Med 2005; 352: 1011- 1023.
- Clark DJ, Cleman MW, Pfau SE, Rollins SA, Ramahi TM, Mayer C et al. Serum complement activation in congestive heart failure. Am Heart J 2001; 141: 684-690
- Fava RA, Gates C, Townes AS. Critical role of periph- eral blood phagocytes and the involvement of comple- ment in tumor necrosis factor enhancement of passive collagen-arthritis. Clin Exp Immunol 1993; 94: 261-266.
- Hsueh W, Sun X, Rioja LN, Gonzalez-Crussi F. The role of the complement system in shock and tissue in- jury induced by tumor necrosis factor and endotoxin. Immunology 1990; 70: 309-314.
- Levine B, Kalman J, Mayer L, Fillit H, Packer M: Ele- vated circulating levels of tumor necrosis factor alpha in severe chronic heart failure. N Engl J Med 1990; 323: 236-241.
- Sharma R, Anker SD: From tissue wasting to cachexia: changes in peripheral blood flow and skeletal muscula- ture. Eur Heart J 2002; 4 (Suppl D):D12-D17.
- Sharma R, Bolger AP, Li W, Davlouros PA, Volk HD, Poole-Wilson PA et al. Elevated circulating levels of inflammatory cytokines and bacterial endotoxin in adults with congenital heart disease. Am J Cardiol 2003; 92: 188-193.
- McMurray J, Abdullah I, Dargie HJ, Shapiro D. In- creased concentrations of tumour necrosis factor in “cachectic” patients with severe chronic heart failure. Br Heart J 1991; 66: 356-358.
- Adams V, Jiang H, Jiangtao Y, Mobius-winkler S, Fiehn E, Linke A et al. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol 1999; 33: 959-965.