ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2020) Volume 31, Issue 5
2-Methacryloyloxyethyl phosphorylcholine polymer can facilitate the reduction in bacterial quantity while maintaining bacterial homeostasis in denture plaque
Akihiro Tsukahara1, Kenji Ikeya1, Hirobumi Morisaki2, Fuminori Iwasa1, Yuuki Inoue3, Miya Fukunishi1, Nana Takahashi1, Hirotaka Kuwata2, Kazuhiko Ishihara3, Kazuyoshi Baba1*
1Department of Prosthodontics, School of Dentistry, Showa University, Tokyo, Japan
2Department of Oral Microbiology and Immunology, School of Dentistry, Showa University, Tokyo, Japan
3Department of Materials Engineering School of Engineering, The University of Tokyo, Tokyo, Japan
- Corresponding Author:
- Kazuyoshi Baba
Department of Prosthodontics
School of Dentistry
Showa University
Tokyo
Japan
Accepted date: August 10, 2020
The infections caused by denture plaque are regarded as a source of serious dental and medical complications in the elderly population. It is important for the treatment of patients with edentulism to understand the pathogens in denture plaque. However, current data are limited and inconclusive. The purpose of this clinical study was to analyse the bacterial composition of the denture plaque and to evaluate the changes in bacterial composition of the denture plaque before and after 2- methacryloyloxyethyl phosphorylcholine (MPC) polymer coating. Ten individuals with maxillary complete dentures participated in this study. Their dentures were treated with poly (MPC-co-Butyl Methacrylate (BMA) -co- 2-methacryloyloxyethyl-4-azidobenzoate (MPAz)) (PMBPAz), and the composition of denture plaque was evaluated by using next-generation sequencing (NGS) after 1 weeks of denture usage. The same procedures were repeated to evaluate the original denture surfaces as a control. We calculated Shannon index (H) and Bray–Curtis index (δAB) with the result of bacterial composition. Students paired t-test was performed to test the effect of the treatment with PMBPAz on the amount of eubacteria and difference in the (H) and (BC) index before and after coating. A total of 25 different genera representing 136 different species/phylotypes were present in at least two subjects at a relative abundance of>0.1%. The (H') index accounted for various values for each patient, and the bacterial constitution had specificity for each patient. On the other hand, the change in the index before and after MPC coating in the same patient did not show any significant difference (UMIN000035554).
Keywords
Bacterial flora, Biomedical polymeric material, Denture plaque, Homeostasis, Oral hygiene.
Introduction
In many countries around the world, advances in medical technology and concomitant health improvements are increasing the elderly population. In Japan, it is expected that 34 million people will be ≥ 65 years of age by 2060 (i.e., 1 in 2.5 of the total population). Considering that oral health status declines with age, the need for dentures is predicted to increase with the increased number of older individuals. To date, research shows that more than 700 types of bacteria have been identified in the human oral cavity, including those that affect the host, ranging from affecting the oral cavity to the whole body.
These bacteria form colonies due to their own growth, which are called plaques; when they form on teeth, they are known as ‘dental plaques.’ In addition, if denture hygiene is not properly maintained, plaques will also form on the surface of the denture, known as ‘denture plaques.’ Denture plaque causes various diseases in the oral cavity, including denture stomatitis and angular cheilitis [1,2]. More importantly, denture plaque is considered a significant risk factor for opportunistic infections and aspiration pneumonia in elderly individuals who wear dentures, in bedridden patients in nursing homes, and in immunocompromised patients [3]. In recent years, the number of deaths in Japan due to pneumonia has increased with the aging population. The latest statistics show that pneumonia is the third leading cause of death in Japan after malignant neoplasms and heart disease [4,5]. In addition, the elderly may not be able to maintain proper denture hygiene for health reasons; this may also be a problem for elderly individuals who cannot move due to illness or other causes. For this reason, infectious diseases such as pneumonia associated with denture plaque are considered to be important health issues for the elderly and are currently receiving attention. At this point, it is easy to imagine how important denture plaque removal is in today's aging society.
However, despite the presence of such disease-causing bacteria in denture plaques, it remains unclear what bacteria are present and to what extent. Many studies have been performed to identify oral bacteria [6]. Furthermore, the maintenance of homeostasis between the oral flora and the host is considered to be an important factor in maintaining health. In general, the oral flora in adults is characterized by high levels of specificity and stability in each person. However, disorders such as administration of antibiotics and contact with foreign substances are known to seriously affect the oral flora [7]. For these reasons, in order to maintain oral homeostasis, the materials used in the oral cavity must not affect any of the host's oral microbiota. Polymers composed of 2- methacryloyloxyethyl phosphorylcholine (MPC) have been shown to inhibit protein adsorption and have excellent affinity for blood and body tissues; further, MPC is recognized as a biomedical polymer material [8-10]. As a biomaterial, MPC polymers have been extensively tested and have been found to be safe. Further, medical devices containing MPC polymers are already used in everyday life and in clinical settings [11-15].
Recently, we investigated the potential of MPC polymers in inhibiting bacterial adhesion on a denture surface using some coating methods, such as dipping, graft polymerization, and polymer poly (MPC-co-n-butyl methacrylate (BMA)-co-2- metahcryloyloxyethyl-4-azidobenzoate (MPAz)) (PMBPAz) coating. Among these coating methods for the poly (methyl methacrylate) (PMMA) surface, the PMBPAz coating (designed using a novel photoreactive monomer bearing a phenylazide group, i.e., MPAz) was found to have the most straightforward and stable manner. Clinically, we showed that the adhesion of denture plaque was suppressed on complete maxillary dentures [16].
In this study, we analyzed the bacterial composition of denture plaque attached to maxillary complete dentures using a next generation sequencer (NGS). Moreover, considering the possibility of microbial substitution by the MPC polymer, we evaluated the changes in bacterial composition and content of the denture plaque before and after the MPC polymer coating at the phylum and genus levels of biological classification.
Materials and Methods
Subject population and sample collection
In this study, patients with complete dentures in the maxillary edentulous region and who were being seen at the Prosthodontics Clinic, Showa University Dental Hospital were included.
In total, 10 (mean age=74.7 ± 7.1 years; seven males, three females) patients participated in the study. This clinical study was conducted in accordance with the Helsinki Declaration and its subsequent amendments. All participants included in the study provided informed consent. The research protocol was approved by the Ethics Committee of Showa University School of Dentistry (2016-030).
Clinical sample collection
Denture plaque on the denture surface was collected as follows. On the first day of the experiment, the denture plaque was mechanically removed from the denture of the patient using a denture brush. Subsequently, the denture plaque was chemically removed using an ultrasonic cleaner and a denture cleaner (labalakD; Sundental Co, Ltd, Osaka, Japan), and the denture plaque adhering to the surface of the denture was thoroughly removed. Then, the denture surface was polished as usual, and the denture was visually inspected to confirm that hardly any plaque was adhered to its surface. In the experimental group, the surface of the denture was also treated with PMBPAz according to the study protocol described above, and the subjects used the denture with the hips for one week. In the control group, dentures were used for the same period of time, but without PMBPAz treatment, and the same evaluation was performed as in the experimental group. During this period, the use of chemical denture cleaners was banned in both counties (Table 1).
| Gender | Mean age | Mean age of denture | Denture cleansing agent usage frequency | Denture cleaning frequency | |
|---|---|---|---|---|---|
| Patient n=10 | Male/ female |
(years) | (month) | ( /week) | ( /day) |
| 7/3 | 74.7 ± 7.1 (min 65: , max: 89) | 24.1 ± 16.6 (min 2: , max:34) | 4.4 ± 2.24 (min 0: , max: 7) | 3.0 ± 1.73 (min 0: , max: 5) |
Table 1: Patient demographics.
Sampling of the denture plaque
Biofilms accumulated on dentures were collected by first rinsing the dentures for 30 s in an ultrasonic bath with distilled water. Then, 50 mL of physiological saline (Otsuka Normal Saline; Otsuka Pharmaceutical Co Ltd., Tokyo, Japan) was added to the pouch holding the dentures and mixed in the ultrasonic bath to detach and resuspend the bacteria adhered to the dentures. Thereafter, this liquid was collected in a 50 mL centrifuge tube, and a centrifugal separator (Himac CT6E; Hitachi Ltd., Japan, Tokyo, Japan) was used to collect denture plaque. The liquid was divided into a precipitate and a supernatant liquid. Finally, the supernatant liquid was drained, and the precipitate was stored at −80°C until DNA extraction.
DNA extraction and sequencing and sequencing data analysis
After denture plaques were collected from the patient and Polymerase chain reaction (PCR), NGS, and quantitative insights into microbial ecology (QIIME) were performed at the J-Bio 21 Center (Nippon Steel &Sumikin Eco-tech Corp., Chiyoda-ku, Japan). The U515F and 926R primer set was used to amplify the V4-V5 region of the bacterial 16S RNA gene [17]. Sequence similarities of 97% were clustered in operational taxonomic units (OTUs) using UCLUST with the furthest algorithm [18]. OTUs related to the bacteria capable of using azo dyes and aniline (as a representative aromatic amine) were analyzed against the KEGG and UniProt databases [19,20].
PMBPAz synthesis and coating
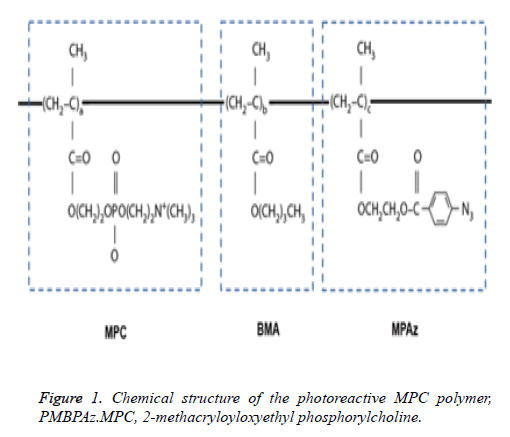
MPC was industrially purified according to previously reported methods and received from the NOF Corp (Tokyo, Japan). PMBPAz containing the MPC polymer was synthesized by the radical polymerization of MPC, BMA, and MPAz in ethanol according to previously described methods [21]. The component compositions of the monomers used were 60%, 30%, and 10% for MPC, BMA, and MPAz, respectively. The structural formula of PMBPAz is shown in Figure 1. The PMBPAz (prepared as described above) was dissolved in ethanol to prepare a 0.5% by weight solution.
Bioinformatics and statistical analysis
The Shannon index represents the degree of diversity prevailing among tested samples; with higher values indicating a higher level of diversity and vice versa. In this study, we used this index to indicate the α-diversity and the diversity within the denture plaque of each patient.
Shannon index (H') = −Σ (Pi) (In Pi)
The Bray–Curtis index (BC index) was used to determine the effect of PMBPAz before and after coating. The BC index is bound between 0 and 1, where 0 indicates that two samples have the same composition and 1 indicates that two samples do not have any common species.
BC index (δAB) = Σ|nAi − nBi| / NA + NB
0 < δAB < 1
The results of the change in number of eubacteria after PMBPAz coating are expressed as mean ± standard deviation values. A Student’s paired t-test was performed to test the effect of the treatment with PMBPAz on the number of eubacteria and the difference in the Shannon index before and after coating (a=0.05; JMP Pro 12; SAS Institute, Inc., Cary, NC, USA). P-values less than 0.05 were considered to be statistically significant.
Results
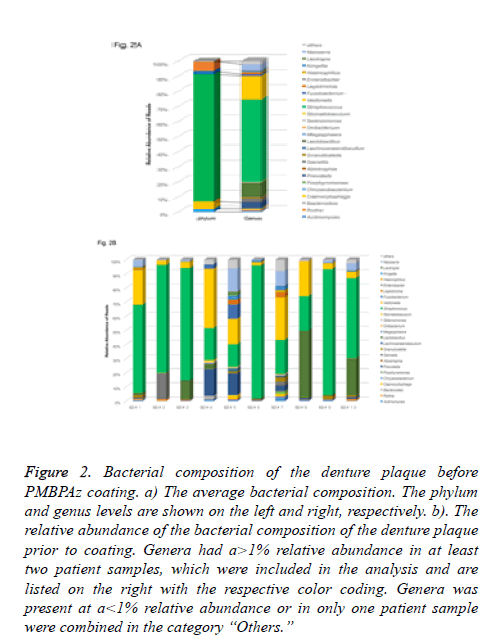
In this study, the analysis of the 16S rRNA gene sequence was processed as described in the Materials and Methods section above. A total of 25 different genera representing 136 different species/phylogenetic types were present in at least two subjects in relative amounts greater than 0.1%. In the microbiota in the denture plaque, the most abundant phylum was Firmicutes (84%), followed by Proteobacteria (6.1%), Bacteroidetes (5.2%), and Actinobacteria (1.9%) in the complete maxillary denture (Figure 2A). However, only two phyla (Firmicutes and Proteobacteria) dominated the communities of denture plaque.
Figure 2: Bacterial composition of the denture plaque before PMBPAz coating. a) The average bacterial composition. The phylum and genus levels are shown on the left and right, respectively. b). The relative abundance of the bacterial composition of the denture plaque prior to coating. Genera had a>1% relative abundance in at least two patient samples, which were included in the analysis and are listed on the right with the respective color coding. Genera was present at a<1% relative abundance or in only one patient sample were combined in the category “Others.”
Among these, the microbiota of denture plaque was predominantly comprised of Firmicutes, followed by Proteobacteria, Bacteroidetes, and either Actinobacteria or Fusobacteria in a decreasing order of relative abundance. At the genus level, the generasStreptococcus, Fusobacteria, and Lactobacillus belonging to the phylum Firmicutes, which occupied the largest proportion of the complete maxillary dentures, were present at a ratio of 54%, 16%, and 9.4%, respectively. In addition, the genera Neisseria (4.0%) belonging to the phyla Proteobacteria, and Prevotella (4.2%) which belongs to the phylum Bacteroides were present (Figure 2B).
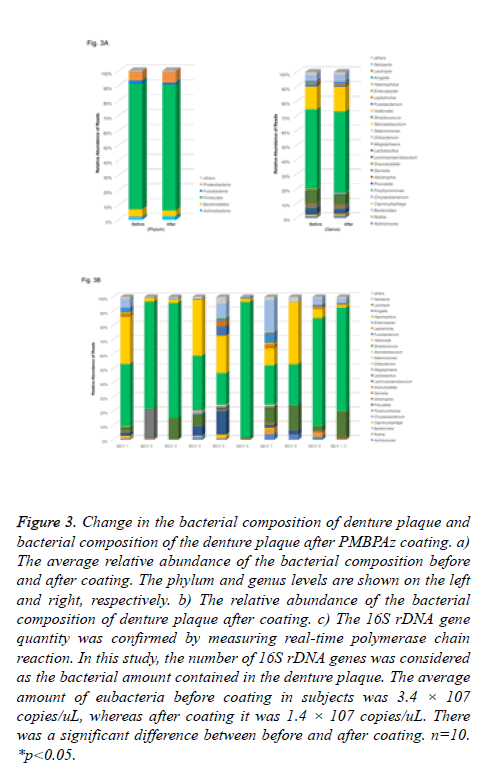
Denture plaque was similarly analyzed before and after PMBPAz coating. The most abundant phylum in the microbiota following PMBPAz coating was Firmicutes (84%), followed by Proteobacteria (7.4%), Bacteroidetes (3.9%), and Actinobacteria (2.4%) in complete maxillary dentures (Figure 3A). At the genus level, the genera Streptococcus, Fusobacteria, and Lactobacillus belonging to the phylum Firmicutes, which occupied the largest proportion in complete maxillary dentures, existed at ratios of 56%, 17%, and 7.5%, respectively. In addition, the genera Neisseria (5.0%) belong to the phylum Proteobacteria, and Prevotella (3.2%) belonging to the phylum Bacteroides, were observed. Moreover, after coating, the proportion of Rothia, Granulicatella, Porphyromonas, Haemophilus, Neisseria, and Gemella bacteria decreased compared with that before coating (Figure 3B). Prior to performing the sequencing analysis, the amount of the eubacteria 16S rDNA gene of each subject was measured using real-time PCR, and the amount of the 16S rDNA gene was confirmed. In this study, the amount of 16S rDNA gene was considered as the amount of bacteria contained in the denture plaque. The average amount of eubacteria before coating in subjects was 3.4 × 107 copies/uL, whereas that after coating was 1.4 × 107 copies/uL(Figure 3C). It was confirmed that the amount of bacteria in the denture plaque decreased following PMBPAz coating. After sequencing analysis, we investigated the change in α-diversity to evaluate the change in the bacterial flora of the denture plaque.
Figure 3: Change in the bacterial composition of denture plaque and bacterial composition of the denture plaque after PMBPAz coating. a) The average relative abundance of the bacterial composition before and after coating. The phylum and genus levels are shown on the left and right, respectively. b) The relative abundance of the bacterial composition of denture plaque after coating. c) The 16S rDNA gene quantity was confirmed by measuring real-time polymerase chain reaction. In this study, the number of 16S rDNA genes was considered as the bacterial amount contained in the denture plaque. The average amount of eubacteria before coating in subjects was 3.4 × 107 copies/uL, whereas after coating it was 1.4 × 107 copies/uL. There was a significant difference between before and after coating. n=10. *p<0.05.
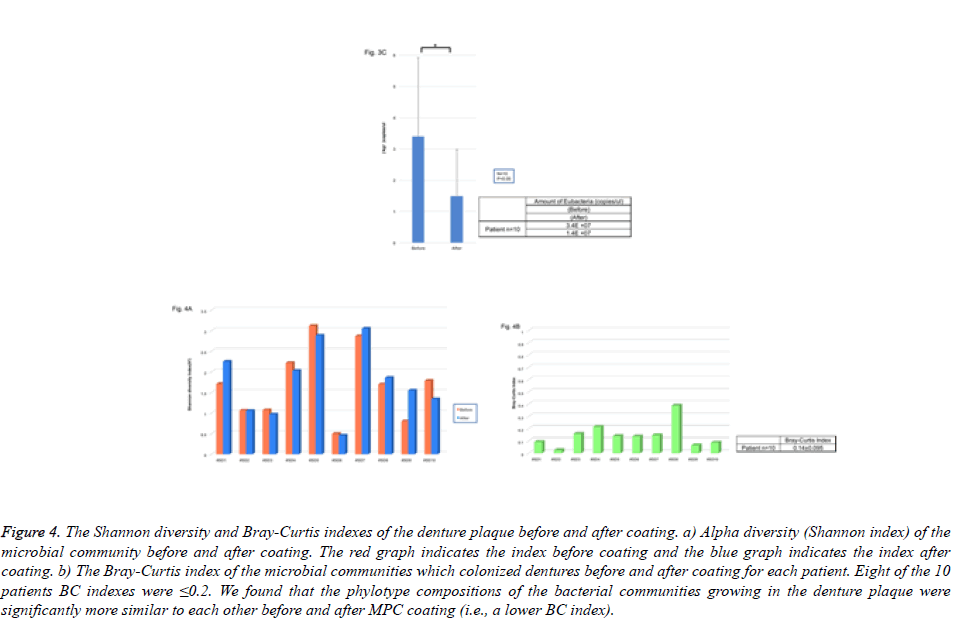
Microbial community richness (Shannon index) at the genus level before coating was on average 1.68 (range, 0.49-3.11) for each sample. On the other hand, microbial community diversity at the genus level after coating was an average of 1.74 (range, 0.45-3.05) for each sample (Figure 4). The Shannon index accounted for various values for each patient, and the bacterial constitution had specificity for each patient. On the other hand, the change in the index before and after PMBPAz in the same patient did not show any significant difference (Figure 4A). To investigate the similarity of bacterial composition before and after coating, we calculated the BC index. Similarity in microbial communities before coating averaged to 0.14 (range, 0.02–0.38) for each sample (Figure 4B). Moreover, 8 of the 10 patients BC indexes were ≤ 0.2. We found that the phylotype compositions of bacterial communities growing in denture plaque were significantly more similar to each other before and after PMBPAz coating (i.e., a lower BC index). Thus, the effect of the MPC coating on the bacterial composition was relatively weak, and it was considered that there was little influence on the homeostasis in the bacterial flora.
Figure 4: The Shannon diversity and Bray-Curtis indexes of the denture plaque before and after coating. a) Alpha diversity (Shannon index) of the microbial community before and after coating. The red graph indicates the index before coating and the blue graph indicates the index after coating. b) The Bray-Curtis index of the microbial communities which colonized dentures before and after coating for each patient. Eight of the 10 patients BC indexes were ≤0.2. We found that the phylotype compositions of the bacterial communities growing in the denture plaque were significantly more similar to each other before and after MPC coating (i.e., a lower BC index).
Discussion
The emergence of high-throughput sequences, such as the next-generation sequencers used in this study, has revolutionized the analysis of microbial ecosystems. With this method, the bacterial flora of denture plaque could be fully analyzed and considered. This was the first study to examine the bacterial flora of denture plaque for maxillary complete dentures. Compared to previous studies, the denture plaques were found to have a relatively high percentage of Firmicutes compared to other sites such as the oral mucosa, tongue, and saliva [22]. Furthermore, at the genus level, Streptococci belonging to the phylum mymicutes accounted for more than about 60% of this group. This bacterium is known as one of the causative bacteria causing dental caries in the oral cavity. The percentage of Streptococcus belonging to this genus was lower in the denture plaque than in the oral cavity. The difference in bacterial composition between the oral cavity and the denture plaque as shown here is considered to be due to the differences in mechanical properties between the oral mucosa and the denture surface. In the oral cavity, formed biofilms are often washed away by the action of secreted saliva. However, denture plaque easily adheres to the resin used for denture production (which was also used in this study) because its surface is porous. Several strains of Streptococcus are very involved in the early stages of plaque formation, and these bacteria can easily attach to the denture surface due to its rough nature. On the other hand, in some specimens, the Neisseria and Prevotella generas belonging to the Proteobacteria and Bacteroidetes phyla, respectively, accounted for 20%-30% of thetotal bacterial flora of the denture plaque. The patients with such a bacterial composition used a limited amount of denture cleanser; further, the patients did not completely clean the dentures and tended to sleep while wearing the dentures. Owing to improper denture cleaning, denture hygiene was substantially affected. Generally, during biofilm formation, one of the first bacterium to adhere to the surface includes Streptococcus. After 24 h, however, the denture plaque matures and contains more types of bacteria, such as an obligate anaerobe. If patients regularly use denture cleansers and frequently clean dentures, although such plaque formation is bound to occur on denture surfaces, bacteria will not be able adhere to denture surfaces. Conversely, poor maintenance of denture hygiene triggers plaque formation by bacteria depending on the process and different bacterial composition among different individuals.
In this study, PMMA was used as the basis material. The MPC polymers with the MPAz units were covalently bound to the substrate. In vitro and in vivo experiments were conducted once with PMBPAz as a coating material. Initially, we examined the ability of PMBPAz coating on PMMA-based dental resin to inhibit bacterial plaque formation in vitro and in vivo [16,23].
As a second step in the clinical study, we investigated the transition of bacteria in terms of volume and composition. As a result, the measurement of the DNA content of eubacteria indicated that the bacterial count decreased after coating. Moreover, we compared the bacteria that changed before and after coating at the genus level. Based on the scores of the BC index, slight changes were observed in the bacterial composition of most bacteria. There were few bacteria that changed in denture plaque, and the bacterial composition remained almost unchanged even after coating.
This was expected due to the properties of the MPC polymer. The MPC polymer used in our study and the PMBPAz prepared by modifying its side chain did not affect the plaques formed on the PMMA surface, although these chemically bond to PMMA, which is a denture material. These facts also can be evaluated from the results of the BC index in this study, i.e., there was no influence on the homeostasis of the bacterial flora formed on the PMMA surface. The widespread use of topical and systemic agents has resulted in a change in bacterial constitution to an extent depending on the effects or side effects. In cases of severe changes, the developed symptoms are referred to as microbial substitution [24,25]. During the outbreak of resistant Staphylococci and in the presence of black hairy tongue and candidiasis in the oral cavity, local or systemic effects might occur. For the maintenance of oral homeostasis of the host, keeping a diversity of the oral microbiota plays an important role. Biomaterials affect the interaction of a biological system. Key features, such as biocompatibility, are fundamental for the development of novel biomaterials in medical and dental fields. The safety of MPC polymer used in this study as a biomaterial was well established. Further, the PMBPAz used in this study caused little change in bacterial constitution and did not affect the homeostasis of denture plaque. In other words, we believe that using MPC polymer; it was possible to reduce only the bacterial quantity while maintaining homeostasis without changing the bacterial composition.
Conclusion
In the present clinical study, we analyzed the bacterial composition of the denture plaque attached to complete maxillary dentures using NGS and evaluated the changes in bacterial composition and content in denture plaque before and after MPC polymer (PMBPAz) coating. As per the valuation of the denture plaque in this study, Firmicutes was the dominant species at the phylum level, accounting for approximately 80% of the total bacterial content, followed by Proteobacteria and Bacteroides. Microbial community richness (Shannon index) at the genus level accounted for various values for each patient, and the bacterial constitution was specific to each patient. The PMBPAz coating decreased the DNA content of the eubacteria in the denture plaque at the cellular level, had little effect on the bacterial constitution, and did not affect the homeostasis of bacterial flora in denture plaque. In summary, the MPC polymer can facilitate the reduction of bacterial quantity while maintaining homeostasis without changing the bacterial composition.
Acknowledgment
The authors thank all of the participants, dentists, and assistants for their participation in this study and the prosthodontics faculty at Showa University, including Professor Matsuo Yamamoto (Department of Periodontology), Professor MasamichiTakami (Department of Dental Pharmacology), and Professor ShoujiHironaka (Department of Special Needs Dentistry, Division of Hygiene and Oral Health) for their support. This study was supported by the Japan Society for the Promotion of Science KAKENHI grants (JSPS KAKENHI; grant numbers 16H07197 and 16H07196).
References
- Budtz-Jorgensen E. Candida-associated denture stomatitis and angular cheilitis. Oral Candidosis 1990; 156-183.
- Hoshi N, Mori H, Taguchi H, Taniguchi M, Aoki H, Sawada T, Kawabata M, Kuwabara A, Oono A, Tanaka K, Hori N, Toyoda M, Kimoto K. Management of oral candidiasis in denture wearers. J Prosthodont Res 2011; 55: 48-52.
- Kulak-Ozkan Y, Kazazoglu E, Arikan A. Oral hygiene habits, denture cleanliness, presence of yeasts and stomatitis in elderly people. J Oral Rehabil 2002; 29:300-304.
- Kenzaka T, Kosami K, Matsuoka Y, Noda A, Kumabe A. The difference between ideal and actual fasting duration in the treatment of patients with aspiration pneumonia: a nationwide survey of clinicians in Japan. Tohoku J Exp Med 2016; 240: 227-233.
- Teramoto S, Fukuchi Y, Sasaki H, Sato K, Sekizawa K, Matsuse T. High incidence of aspiration pneumonia in community and hospital-acquired pneumonia in hospitalized patients: a multicenter, prospective study in Japan. J Am Geriatr Soc 2008; 56: 577–579.
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005; 43: 5721-5732.
- Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010; 156: 3216–3223.
- Ishihara K, Aragaki R, Ueda T, Watenabe A, Nakabayashi N. Reduced thrombogenicity of polymers having phospholipid polar groups. J Biomed Mater Res 1990; 24: 1069-1077.
- Ishihara K, Ziats NP, Tierney BP, Nakabayashi N, Anderson JM. Protein adsorption from human plasma is reduced on phospholipid polymers. J Biomed Mater Res 1991; 25: 1397-1407.
- Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipid polymers reduce protein adsorption? J Biomed Mater Res 1998; 39:323-330.
- Ishihara K. Bioinspired phospholipid polymer biomaterials for making high performance artificial organs. Sci Technol Adv Mater 2000; 1: 131-138.
- Iwasaki Y, Ishihara K. Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci Technol Adv Mater 2012; 13: 064101.
- Snyder TA, Tsukui H, Kihara S, Akimoto T, Litwak KN, Marina V Kameneva, Yamazaki K, William R Wagner. Preclinical biocompatibility assessment of the EVAHEART ventricular assist device: coating comparison and platelet activation. J Biomed Mater Res 2007; 81: 85-92.
- Myers GJ, Johnstone DR, Swyer WJ, McTeer S, Maxwell SL, Chris Squires, Steve N Ditmore, Clarie V Power, Lance B Mitchell, Jan E Ditmore, Larry D Aniuk, Greg M Hirsch, Karen J Buth. Evaluation of Mimesys phosphorylcholine (PC)-coated oxygenators during cardiopulmonary bypass in adults. J Extra Corpor Technol 2003; 35: 6-12.
- Moro T, Takatori Y, Ishihara K, Konno T, Takigawa Y, Matsushita T, Ung-il Chung, Nakamura K, Kawaguchi H. Surface grafting of artificial joints with a biocompatible polymer for preventing periprosthetic osteolysis. Nat Mater 2004; 3: 829-835.
- Ikeya K, Iwasa F, Inoue Y, Fukunishi M, Takahashi N, Ishihara K, Baba K. Inhibition of denture plaque deposition on complete dentures by 2-methacryloyloxyethyl phosphorylcholine polymer coating: A clinical study. J Prosthet Dent 2018; 119: 67-74.
- Cai L, Ye L, Tong AH, Lok S, Zhang T. Biased diversity metrics revealed by bacterial 16S pyrotags derived from different primer sets. PLoS One 2013; 8: e53649.
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010; 26: 2460-2461.
- Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 2014; 42: D199-D205.
- UniProt Consortium. Ongoing and future developments at the universal protein resource. Nucleic Acids Res 2011; 39: D214-D219.
- Fukazawa K, Ishihara K. Synthesis of photoreactive phospholipid polymers for use in versatile surface modification of various materials to obtain extreme wettability. ACS Appl Mater Interfaces 2013; 5: 6832-6836.
- Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Dirk Gevers, Curtis Huttenhower, Jacques Izard. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol 2012; 13: R42.
- Fukunishi M, Inoue Y, Morisaki H, Kuwata H, Ishihara K. A polymethyl methacrylate-based acrylic dental resin surface bound with a photoreactive polymer inhibits accumulation of bacterial plaque. Int J Prosthodont 2017; 30: 533–540.
- Bicmen C, Doluca M, Gulat S, Gunduz AT, Tuksavul F. Species level identification and antifungal susceptibility of yeast isolated from various clinical specimens and evaluation of Integral System Yeasts Plus. New Microbiol 2012; 35: 327-334.
- Mohamadi J, Motaghi M, Panahi J, Havasian MR, Delpisheh A, Azizian M. Anti-fungal resistance in Candida isolated from oral and diaper rash candidiasis in neonates. Bioinformation 2014; 10: 667–670.