ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 2
Preparation process of paclitaxel liposomes and its anti-proliferative effect on human gastric cancer MGC803 cells.
| Huiguang Wang1, Yan Xue1, Weiqing Di1, Liang Sun1, Rongliang Chen2* 1Liaoning NuoKang Bio-Pharmaceutical Co., LTD, Shenyang 110011, P.R. China 2Liaoning Medical Device Test Institute, Shenyang 110179, P.R. China |
| Corresponding Author: Rongliang Chen Department of Clinical Laboratory Qingdao University, PR. China |
| Accepted: January 20, 2016 |
To study the preparation process of paclitaxel liposomes, and determine its effect on human gastric cancer MGC803 cells. Orthogonal experiment was employed to study the preparation process of paclitaxel liposomes, and MTT assay was used to detect its effect of paclitaxel liposomes on cells. Changes in cell structure were observed by light microscopy, while cell cycle distribution of MGC803 cells was detected by flow cytometry. Factors influencing the encapsulation efficiency of paclitaxel liposomes were, in the order of importance, proportion of hydrogenated phospholipid to paclitaxel, weight proportion of phospholipid to cholesterol, and rotary evaporation temperature. Furthermore, optimal preparation process of paclitaxel liposomes was identified as phospholipid to cholesterol weight ratio of 20:4.28, hydrogenated phospholipid to paclitaxel weight ratio of 20:1, and rotary evaporation temperature of 60°C. 24 ~ 72 h after treatment of MGC803 cells, different doses of paclitaxel test solutions could all reduce MGC803 cell viability. Flow cytometry results showed that paclitaxel had a very significant effect on MGC803 cell cycle distribution. Under electron microscope, paclitaxel-treated MGC803 cells presented ill-defined boundaries, reduced glycogen, swollen organelles, liquefactive necrosis and liquefaction degeneration of cytoplasm. Besides, number of mitochondria decreased, with residual organelle debris visible only. Paclitaxel liposomes have an inhibitory effect on gastric cancer MGC803 cells without suppressing normal cells at tested doses.
Keywords |
||||||
| Paclitaxel, Liposome, MGC803 cell, Flow cytometry. | ||||||
Introduction |
||||||
| Taxus cuspidate Sieb.et Zucc. is a plant in the family Taxaceae S.F. Grey, order Taxales, class Coniferopsida of subdivision Gymnospermae, which is also known as Zishan, Chibisong, Mishu, Kuanyezishan, etc. It is an ancient tertiary plant species, which is a relict plant of glacial period. As a coniferous arbor or shrub, it can grow to 20 m tall, with a diameter at breast height up to 40 cm. Its barks are reddishbrown, and shallowly fissured. | ||||||
| The tree is mainly distributed in the hills and valleys of the Yangtze River Basin, Lingnan mountains and Henan, Shaanxi (Qin Mausoleum), Gansu, Taiwan and other places [1]. It is generally believed that there are 11 species of plants in the genus Taxus of family Taxaceae where Taxus cuspidate Sieb.et Zucc. belongs. China is rich in Taxus resources, where Taxus chinensis, Taxus wallichiana Zucc., Taxus yunnanensis, Taxus cuspidate Sieb.et Zucc. and Taxus chinensis var. mairei, a total of 4 species and 1 variant, can be found [2]. | ||||||
| Studies on the chemical constituents of Taxus began since the mid-1980s, and now about 500 types of taxanes have been isolated and identified from the Taxus plants [3-8]. Extensive research has been done on paclitaxel at home and abroad. According to modern pharmacological studies, paclitaxel has a potent inhibitory activity on a variety of in-vitro and transplanted tumors, which can markedly inhibit human tumors, breast cancer, colon cancer, lung cancer and melanoma xenografts, and has certain therapeutic effects on gastric cancer, leukemia and lung cancer as well [9,10]. | ||||||
| Nevertheless, due to the extremely low water solubility of paclitaxel, it cannot be absorbed orally, which seriously affects its clinical application. Thus, in this study, the preparation process of paclitaxel liposomes is investigated and its antigastric cancer cell action is studied, in order to provide possibilities to its clinical application. | ||||||
Material and Methods |
||||||
Instruments |
||||||
| RE-52CS rotary evaporator (Shanghai Zhenjie Laboratory Equipment Co., Ltd.). CO-150 CO2 incubator (NBS, USA); inverted fluorescence microscope (OLYMPUS, Japan); clean bench (Suzhou Purification Equipment Factory); ELX 800 microplate reader (BioTEK, USA); FACSAria flow cytometry (BD, USA). | ||||||
Reagents |
||||||
| RPMI1640 (Gibco); FBS (Hangzhou Sijiqing Bioengineering Materials Co., Ltd.); MTT, DMSO and PI (Nanjing KeyGEN Biotech Co., Ltd.). Human gastric cancer MGC803 cells were purchased from China Medical University. | ||||||
Results |
||||||
Thin film-ultrasonic preparation of paclitaxel liposomes |
||||||
| Phospholipid and cholesterol were accurately weighed according to the proportion of prescriptions, added with ethyl ether solution and stirred until completely dissolved, then rotary evaporated under reduced pressure in a 35°C water bath to remove the ethyl ether, so that the film material formed a uniform lipoid film on the beaker wall. Meanwhile, prescribed amount of paclitaxel sample was dissolved in ethyl ether, added with lipoid solution, mixed uniformly by ultrasonic oscillation, and evaporated under reduced pressure. Then, nitrogen was blown into the flask at a 1.5 × 106 Pa pressure several cycles to give paclitaxel lipidosomes. | ||||||
Determination of paclitaxel liposome encapsulation efficiency |
||||||
| Appropriate amount of Sephadex G-50 (0.7 cm × 15 cm) which was presoaked in phosphate buffer (pH=7.4) for 12 h and equilibrated was taken to prepare gel column. 2 mL of paclitaxel liposomes was accurately injected into column, and eluted with phosphate buffer at a flow rate of 0.5 ml/min. Eluent was collected, and changes in absorbance of various fractions were measured at 230 nm. Results revealed that the liposomes were completely eluted between about 4-25 mL, while the drug was completely eluted between about 32-65 mL, presenting good resolution between the two. Paclitaxel liposomal fraction was collected, added with ethyl etheranhydrous ethanol-aqueous solution to damage the liposomal membrane, fully shaken to break down emulsion, and then evaporated in a water bath. The residue was diluted in anhydrous ethanol to a constant volume, and absorbance was measured at 230 nm by colorimetry, followed by calculation of liposome encapsulation efficiency. | ||||||
| Encapsulation efficiency En%=Ce/Ct × 100%, where Ce: amount of paclitaxel encapsulated into liposomes; Ct: total amount of paclitaxel in liposomes. | ||||||
Design of orthogonal factors and levels |
||||||
| Based on preliminary experiment and relevant literature, phospholipid to cholesterol proportion, hydrogenated phospholipid to paclitaxel proportion and high pressure homogenization cycles were selected as three factors among factors influencing major quality indices of paclitaxel liposomess such as particle size, distribution, encapsulation efficiency and stability, each of which had three levels. Screening was performed using orthogonal table L9 (33). The factors and levels are shown in Table 1. | ||||||
| As can be seen from Table 2, under the factors and levels adopted in this experiment, the importance of factors influencing the encapsulation efficiency of paclitaxel liposomes were: B>A>C, i.e. hydrogenated phospholipid to paclitaxel proportion >phospholipid to cholesterol weight ratio >rotary evaporation temperature. Range analysis showed that under the experimental conditions, the optimal preparation process of paclitaxel liposomes was A3B1C3, i.e. phospholipid to cholesterol weight ratio of 20:4.28, hydrogenated phospholipid to paclitaxel weight ratio of 20:1, and rotary evaporation temperature of 60°C. | ||||||
Cell cultivation |
||||||
| MGC803 gastric cells were seeded in a culture flask filled with 10% FBS-containing RPMI 1640 medium, cultured under 5% CO2, 37°C conditions, and harvested in the logarithmic phase for experiment. | ||||||
MTT assay of MGC803 cell growth inhibition |
||||||
| Exponential phase MGC803 cells were prepared into a 4 × 104/ml single cell suspension with 10% FBS-containing RPMI 1640, seeded in 96-well plates at 180 μL per well, and cultured for 24 h, then added with experimental concentrations of paclitaxel test solutions (0.1 mg/ml, 0.2 mg/ml, 0.4 mg/ml). Five parallel wells were set up for each group, and control group was added with an equivalent volume of culture medium. After culturing in a 5% CO2 incubator for additional 24 h, 48 h and 72 h, each well was added with 20 μL of 5 mg/ml MTT, and incubated for 4 more h. Then supernatant was discarded, 150 μL of DMSO was added, and the plates were shaken for 10 min. After dissolving the crystals, optical absorbance of each well was measured at 570 nm with a microplate reader. | ||||||
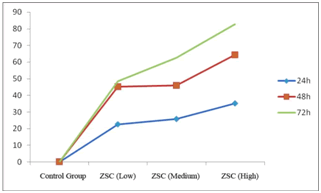
| MTT assay results showed that after treatment of MGC803 cells for 24 ~ 72 h, different doses of paclitaxel test solutions (0.1 mg/ml, 0.2 mg/ml, 0.4 mg/ml) could all reduce the viability of MGC803 cells. With increasing doses, cell viability declined continuously. At the same concentration, cell viability declined continuously over time. MGC803 inhibition rate reached 82.7% in the high-dose group, as shown in Figure 1. | ||||||
FCM analysis of cell cycle distribution |
||||||
| MGC803 cells treated with different concentrations of drugs and control medium for 72 h were collected separately, uniformly scattered, washed twice with PBS, and fixed overnight in 70% cold ethanol. Then, the cells were washed twice with PBS, PI-stained, ice bathed for 30 min, and sampled for cell cycle analysis on flow cytometer. | ||||||
| Flow cytometry results showed that paclitaxel had a very significant effect on cell cycle distribution of MGC803 cells. Compared with the control group, the proportion of S phase MGC803 cells increased from 37.57% in the control group to 58.37% in the treatment group with increasing paclitaxel concentration, while the proportion of G2/M phase cells decreased significantly from 19.98% in the control group to 4.11% in the treatment group. This suggested that paclitaxel could induce S-phase arrest of MGC803 cells. Compared with the control group, G2/M phase cells decreased, whereas S phase cells increased in percentage, presenting a marked timedose- effect relationship, as shown in Table 3. | ||||||
TEM observation |
||||||
| After trypsinization, 1 × 107 of well growing MGC803 cells (adherent, morphologically normal, having agranular cytoplasm with fast cytokinesis) were collected, fixed in 2.5% glutaraldehyde, and later in 2% osmium tetroxide for 30 min. Then, the cells were washed with 1% PBS, fixed in osmic acid, routinely dehydrated, impregnated, embedded in epoxy resin, cut into ultrathin sections with LKB ultramicrotome and stained, followed by observation and photography under TEM. | ||||||
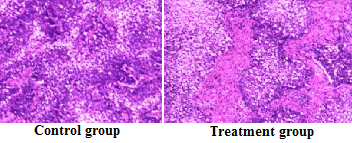
| At high magnification, 48 h after treatment of human gastric cancer MGC803 cells with paclitaxel, the cells in the control group: presented rich microvilli, clear boundaries, a large number of cytoplasmically visible mitochondriaintra, clear ridge structure, a small amount of rough endoplasmic reticula, Golgi complexes and lysosomes. Cells in the paclitaxel treatment groups: had ill-defined boundaries, reduced glycogen, swollen organelles, liquefactive necrosis and liquefaction degeneration of cytoplasm. Besides, number of mitochondria decreased, with residual organelle debris visible only, as shown in Figure 2. | ||||||
Discussion |
||||||
| Liposomes have a biofilm-like bilayer structure that is composed of phospholipids and cholesterol. Through years of research, Rymen et al. successfully applied liposomes in the pharmaceutical industry as a carrier in 1971, since when liposomes have been widely used in the pharmaceutical area as a novel carrier. Compared with other carriers, liposomes have cellular affinity, targeting property and sustained-release characteristics. Moreover, owing to good encapsulation efficiency, liposomes can markedly reduce drug toxicity while improving stability. | ||||||
| Depending on the nature, liposomes can generally be divided into two major categories: ordinary liposomes and special liposomes. The so-called ordinary liposomes refer to those without any modification; such liposomes are readily absorbed by the reticuloendothelial system, thus drugs suitable for reticuloendothelial system uptake can be encapsulated with ordinary liposomes [11]. Special liposomes, on the other hand, refer to modified liposomes, including the long-circulating liposomes modified with PEG on the surface [12], immunoliposomes with antibodies on the surface [13], magnetoliposomes added with some harmless tiny ferromagnetic substances [14], as well as cationic liposomes with cations on the surface. Anticancer drugs encapsulated with cationic liposomes selectively reach the tumor vascular endothelial cells to inhibit angiogenesis, thereby depriving the tumor cells of their nutritional source [15]. | ||||||
| In this study, paclitaxel liposomes are prepared by thin filmultrasonic approach. Among factors influencing major quality indices of paclitaxel liposomess such as particle size, distribution, encapsulation efficiency and stability, phospholipid to cholesterol proportion, hydrogenated phospholipid to paclitaxel proportion and high pressure homogenization cycles are determined as three factors. Degree of importance of factors influencing the encapsulation efficiency of paclitaxel liposomes are verified through orthogonal test to be hydrogenated phospholipid to paclitaxel proportion, phospholipid to cholesterol weight ratio, and rotary evaporation temperature in the descending order. Furthermore, optimal preparation process of paclitaxel liposomes is identified as phospholipid to cholesterol weight ratio of 20:4.28, hydrogenated phospholipid to paclitaxel weight ratio of 20:1, and rotary evaporation temperature of 60°C. | ||||||
Conclusion |
||||||
| In the experiment on inhibitory effect of paclitaxel on gastric cancer cell activity, MTT assay reveals that different doses of paclitaxel test solutions are all able to reduce the viability of MGC803 cells. With increasing doses, cell viability declines continuously. Flow cytometry results demonstrate that paclitaxel has a very significant effect on MGC803 cell cycle distribution, which can induce S-phase arrest of MGC803 cells. The specific mechanisms need further study. | ||||||
Tables at a glance |
||||||
|
||||||
Figures at a glance |
||||||
|
||||||
References |
||||||
|