ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2013) Volume 24, Issue 3
Positive inotropic activity induced by anestradiol derivative in isolated rat heart model.
1Laboratory of Pharmaco-Chemistry, Faculty of Chemical Biological Sciences, University Autonomous of Campeche, Av. Agustín Melgar s/n, Col Buenavista C.P.24039 Campeche Cam., México.
2Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional. Prol. Carpio y Plan de Ayala s/n Col. Santo Tomas, México, D.F. C.P. 11340.
3Facultad de Nutrición,Universidad Autónoma de Veracruz, Médicos y Odontologos s/n C.P. 91010, Unidad del Bosque Xalapa Veracruz, México.
- *Corresponding Author:
- Figueroa V. Lauro
Laboratory of Pharmaco-Chemistry
Faculty of Chemical Biological Sciences
Universidad Autonóma de Campeche
Av. Agustín Melgar s/n
Col Buenavista C.P.24039 Campeche Cam.
México.
Accepted date: March 27 2013
itation : Figueroa V. Lauro, Díaz C. Francisco, García C. Elodia, Pool G. Eduardo, López R. Maria, Rosas-Nexticapa M3, Mendoza- López R3, May G. Ivan1, Sarao Ana1. Positive inotropic activity induced by anestradiol derivative in isolated rat heart model. Biomedical Research 2013; 24 (3): 289-297.
There are studies which indicate that some steroid derivatives have inotropic activity; nevertheless, there is scarce information about of themolecular mechanism involved at cardiovascular level. Therefore, in this study anestradiol derivative was synthetized with the objective of evaluating its inotropic activity. In the first stage, the Langendorff technique was used to measure changes on perfusion pressure and coronary resistance in an isolated rat heart model in absence or presence of estradiol and its derivative.In second stage, the inotropic activity of estradiol derivative was evaluated by measuring left ventricular pressure in absence or presence of following compounds; tamoxifen, prazosin, metoprolol, indomethacin and nifedipine. The results showed that the estradiol derivative significantly increases the perfusion pressure and coronary resistance in comparison with estradiol and the control conditions. Additionally, other data indicate that estradiol derivative increase left ventricular pressure in a dose-dependent manner [0.01 nM to 100 nM]; nevertheless, this phenomenon was significantly inhibited by tamoxifen at a dose of 1 nM. In conclusion, experimental data suggest that, inotropic activity positively induced by estradiol derivative on the left ventricular pressure may involve estrogen receptor activation.
Keywords
Estradiol derivative, Langendorff, inotropic activity.
Introduction
Some reports indicate that congestive heart failure (CHF) is a main cause of death in patients with heart disease [1- 3]. Several drugs have been used for the treatment of CHF such as the digitalis glycosides. Unfortunately, the use of these agents is limited by their narrow therapeutic window and their propensity to cause life-threatening arrhythmias [4,5]. In this sense, there has been a resurgence of interest in cardiotonic steroids derivatives, it is important to mention that these molecules exert a large number of effects in cardiac tissue [6,7]. For example, the strophanthidin (steroid derivative) increase the force of contraction by changes in the calcium levels [8,9]. In addition, there are studies that show the synthesis of a steroid derivative (F90927) which exerts a positive inotropic activity in cardiac muscle via activation of the L-type Ca2+ channel[10]. Additionally, a series of steroid derivatives[ 11, 12] were synthesized which showed a positive inotropic effect, mainly by inhibition of Na+, K+- ATPase. Nevertheless, other reports indicate that 14-β hydroxyprogesterone[13] increases the contractility of isolated cardiac tissue via glycoside receptor. Additionally, it is important to mention that recently anestradiolethylenediamine derivative was synthetized which induce increase the perfusion pressure and vascular resistance via activation of the L-type Ca2+ channel [14]. All these data show that several steroid derivatives induce inotropic effects in the cardiovascular system; nevertheless, the cellular site and molecular mechanism involved in its inotropic activity are very confuse, perhaps this phenomenon is due to differences in the chemical structure of the steroid derivatives. Therefore, data information is needed to characterize the activity induced by steroid derivatives at cardiovascular level. To provide this information, the present study was designed to investigate the effects of an estradiol derivative on perfusion pressure and vascular resistance in isolated rat hearts using the Langendorff technique. In addition, to evaluate the molecular mechanism involved in the inotropic activity induced by the steroidderivative on left ventricular pressure the following compounds were used as pharmacological tools; tamoxifen [antagonist of estrogen receptor][15], prazosin [α1adrenoreceptor antagonist][16], metoprolol [β1 selective receptor blocker][17], nifedipin[antagonist of calcium- channel] [18] and indomethacin[prostaglandin synthesis inhibitor][19].
Material and Methods
Chemical synthesis
The compounds evaluated in this study were purchased from Sigma-Aldrich Co., Ltd. The melting points for the different compounds were determined on an electrothermal (900 model). Infrared spectra (IR) were recorded using KBr pellets on a Perkin Elmer Lambda 40 spectrometer. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Varian VXR-300/5 FT NMR spectrometer at 300 and 75.4 MHz in CDCl3 using tetramethylsilane (TMS) as internal standard. Electron impact mass spectrometric (EIMS) spectra were obtained with a Finnigan Trace GCPolaris Q. spectrometer. Elementary analysis data were acquired from a Perkin Elmer Ser. II CHNS/0 2400 elemental analyzer.
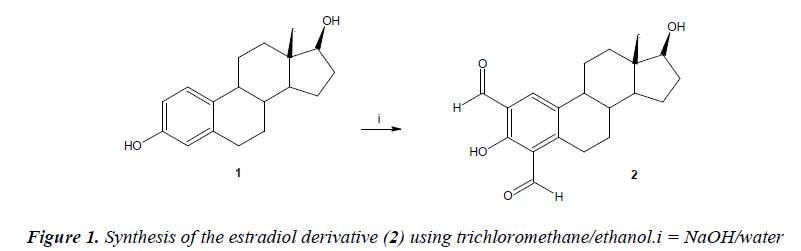
Synthesis of 3,17-Dihydroxy-13-methyl-7,8,9,11,12,13,- 14,15,16,17-decahydro-6H-cyclopenta[a]-phenanthrene- 2,4-dicarbaldehyde (4)
A solution of estradiol (100 mg, 0.37 mmol), sodium hydroxide (100 mg, 2.5 mmol), water (20 ml) andtrichloromethane (20 ml) in 30 ml of ethanol was gently refluxed for 12 h, and then cooled to room temperature. The reaction mixture was evaporated to a smaller volume, diluted with water, and extracted with chloroform. The organic phase was evaporated to dryness under reduced pressure. After, water was added (30 ml) to the mixture obtained. Then theprecipitate was washed 8 times with water and dried at 100°C.
Biological method
All experimental procedures and protocols used in this investigationwere reviewed and approved by the Animal Care and Use Committee of University Autonomous of Campeche (UAC) andwere in accordance with the Guide for the Care and Use of Laboratory Animals[20]. Male rats (Wistar; weighing 200-250 g) were obtained from UAC.
Reagents
All drugs were dissolved in methanol and different dilutions were obtained using Krebs-Henseleit solution (£ 0.01%, v/v).
Langendorff technique
Briefly, the male rat (200 - 250 g) was anesthetized by injecting them with pentobarbital at a dose rate of 50 mg/Kg body weight. Then the chest was opened, and a loose ligature passed through the ascending aorta. The heart was then rapidly removed and immersed in ice cold physiologic saline solution. The heart was trimmed of non-cardiac tissue and retrograde perfused via a noncirculating perfusion system at a constant flow rate. It is important to mention that perfusion medium was the Krebs- Henseleit solution (pH 7.4, 37°C) composed of (mmol); 117.8 NaCl; 6 KCl; 1.75 CaCl2; 1.2 NaH2PO4; 1.2 MgSO4; 24.2 NaHCO3; 5 glucose and 5 sodium pyruvate. The solution was actively bubbled with a mixture of O2/CO2 (95:5). The coronary flow was adjusted with a variable speed peristaltic pump. An initial perfusion rate of 15 ml/min for 5 min was followed by a 25 min equilibration period at a perfusion rate of 10 ml/min. All experimental measurements were done after this equilibration period.
Induction of congestive heart failure (CHF)
CHF was development mainly of method previous reported [21], in this process the pentobarbital (100/kg mg) was administered through of cannula inserted in the aorta to induce CHF.
Perfusion pressure
Evaluation of measurements of perfusion pressure changes induced by drugs administration in this study were assessed using a pressure transducer connected to the chamber where the hearts were mounted and the results entered into a computerized data capture system (Biopac).
Inotropic activity
Contractile function was assessed by measuring left ventricular developed pressure (LVdP), using a saline-filled latex balloon (0.01 mm, diameter) inserted into the left ventricle via the left atrium. It is important to mention that latex balloon was bound to cannula which was linked to pressure transducerthat was connected with the MP100 data acquisition system.
Biological evaluation
First stage
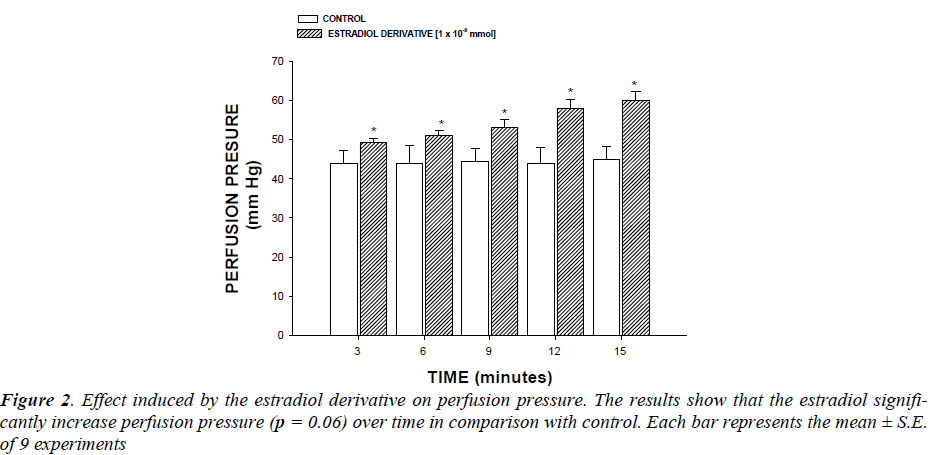
Effect induced by the estradiol derivative on perfusion pressure : Changes in perfusion pressure as a consequence of increases in time (3-15 min) in absence (control) or presence of the estradiol derivative at a concentration of 1 ×10-9 mmol were determined. The effects were obtained in isolated hearts perfused at a constant-flow rate of 10ml/min.
Evaluation of effects exerted by the estradiol derivative on coronary resistance
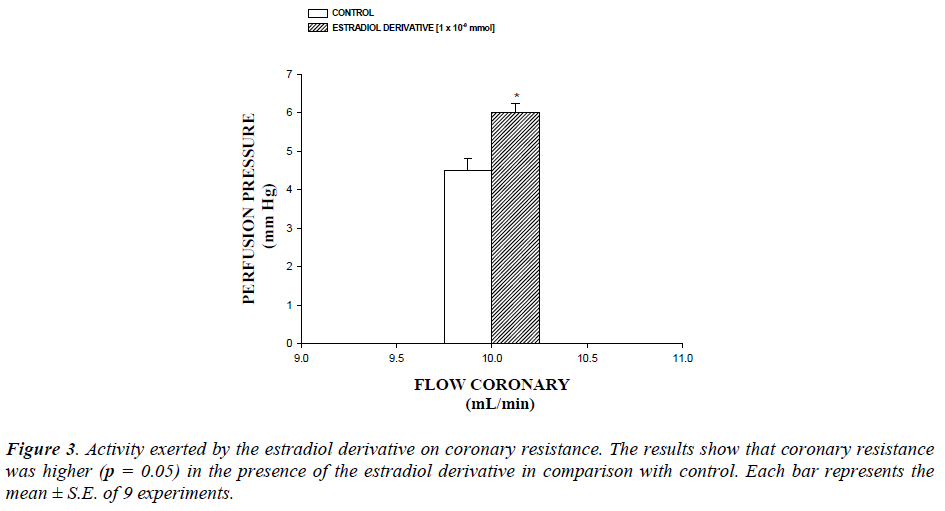
The coronary resistance in absence (control) or presence of the estradiol derivative at a concentration of 1 × 10-9 mmol was evaluated. The effects were obtained in isolated hearts perfused at a constant flow rate of 10 ml/min. Since a constant flow was used changes in coronary pressure reflects the changes in coronary resistance.
Second stage
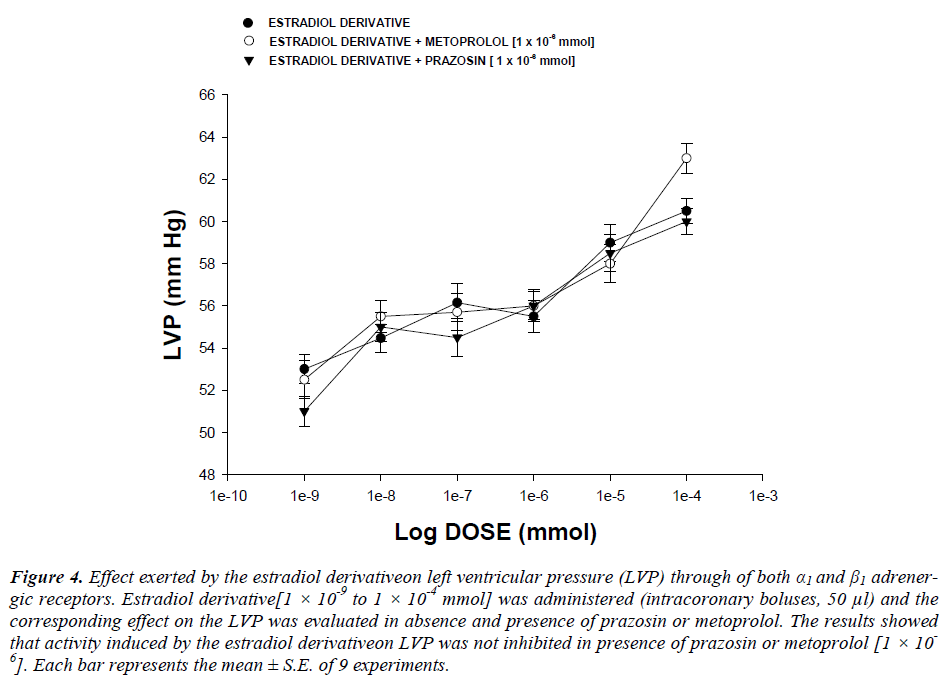
Effect exerted by the estradiol derivative on left ventricular pressure in the presence of both α1or β1adrenergic blockers : The boluses (50 μl) of the estradiol derivative [1 ×10-9 to 1 ×10-4 mmol] were administered and the corresponding effect on the left ventricular pressure was evaluated. It is important to mention that the bolus injection administered was done in the point of cannulation. The dose-response curve (control) was repeated in the presence of prazosin or metoprolol at a concentration of 1 ×10-6 mmol (duration of preincubation with prazosin was by a 10 min equilibration period).
Effects of the estradiol derivative on left ventricular pressurethrough the calcium channel: Intracoronary boluses (50 μl) of the estradiol derivative [1 ×10-9 to 1 ×10-4 mmol] were administered and the corresponding effect on the left ventricular pressure was evaluated. The dose-response curve (control) was repeated in the presence of nifedipine at a concentration of 1 ×10-6 mmol (duration ofpreincubation with nifedipine was by a 10 min equilibration period).
Effects induced by the estradiol derivative on left ventricular through of prostanglandin synthesis: The boluses (50 μl) of the estradiol derivative [1 ×10-9 to 1 ×10-4 mmol] were administered and the corresponding effect on the left ventricular pressure was evaluated. The dose-response curve (control) was repeated in the presence of indomethacin at concentration of 1 ×10-6 mmol (duration of preincubation with indomethacin was by a 10 min equilibration period).
Effects induced by the estradiol derivative on left ventricular pressure through estrogen receptors: Intracoronary boluses (50μl) of the estradiol derivative (1 ×10-9 to 1 ×10-4 mmol) were administered and the corresponding effect on the left ventricular pressure was determined. The dose-response curve (control) was repeated in the presence of tamoxifen at a concentration of 1 ×10-6 mmol (duration of preincubation with tamoxifen was by a 10 min equilibration period).
Statistical analysis
The obtained values are expressed as average ± SE, using each heart as its own control. The data obtained were put under an analysis of variance (ANOVA) using the Bonferroni correction factor [22]. The differences were considered significant when p was equal or smaller than 0.05.
Results
Chemical synthesis
The yielding of estradiol derivative (Figure 1) was of 75% with melting point of 150-152°C. In addition, the spectroscopic analyses show signals for IR (Vmax, cm-1) at 3380 and 3300. In addition, the chemical shifts of the spectroscopic analyses of 1H NMR (Table 1) and 13C NMR (Table 2) for the estradiol derivative are showed down. Finally, the results of mass spectroscopy (MS) (70 ev) shown; m/z 327.14 [M+] 310.39, 286.16, 163.04. Additionally, the elementary analysis data for the estradiol derivative (C20H23O4) were calculated (C, 73.37; H, 7.08; O, 19.55) and found (C, 73.35; H, 7.02).
Biological activity
First stage : In this study, the activity induced by an estradiol derivative on perfusion pressure and coronary resistance in the isolated rat heart was evaluated. The results obtained from changes in perfusion pressure as a consequence of increases in the time (3 to 15 min) in absence (control) or in presence of estradiol derivative (Figure 2, see), showed that the estradiol derivative [1 ×10-9 mmol] significantly increase the perfusion pressure (p = 0.06) in comparison with the control conditions.
Additionally, another result showed that coronary resistance, calculated as the ratio of perfusion pressure at coronary flow assayed (10 ml/min) was higher in the presence of estradiol derivative than in control conditions (p = 0.05) at a concentration of 1 ×10-9 mmol (Figure 3, see).
Second stage : The resultsshowedthat the estradiol derivative increase the left ventricular pressure in a dose dependent manner [1 ×10-9 to 1 ×10-4 mmol] and this effect was not inhibited in presence of prazosin or metoprolol (Figure 4, see) drugs at a concentration of 1 ×10-6 mmol.
Figure 4: Effect exerted by the estradiol derivativeon left ventricular pressure (LVP) through of both α1 and β1 adrenergic receptors. Estradiol derivative[1 × 10-9 to 1 × 10-4 mmol] was administered (intracoronary boluses, 50 μl) and the corresponding effect on the LVP was evaluated in absence and presence of prazosin or metoprolol. The results showed that activity induced by the estradiol derivativeon LVP was not inhibited in presence of prazosin or metoprolol [1 × 10-6]. Each bar represents the mean ± S.E. of 9 experiments.
On the other hand, other experiments showed that estradiol derivative increase the left ventricular pressure in a dose dependent manner [1 ×10-9 to 1 ×10-4 mmol] and this effect was not inhibited in presence of nifedipin or indomethacin drugs(Figure 5 and 6, see) at a concentration of 1 ×10-6 mmol.
Figure 5: Activity exerted by the estradiol derivative on left ventricular pressure (LVP) through L-type of calcium channel. Intracoronary boluses (50 μl) of the estradiol derivative [1 × 10-9 to 1 × 10-4 mmol] were administered in absence and presence of nifedipine [1 × 10-6]. The results showed that effect induced by the estradiol derivative on perfusion pressure in presence of nifedipine was not inhibited. Each bar represents the mean ± SE of 9 experiments.
Figure 6: Effects induced by the estradiol derivative on left ventricular pressure (LVP) through prostanglandin synthesis. Intracoronary boluses (50 μl) of the estradiol derivative [1 × 10-9 to 1 × 10-4 mmol] were administered in absence and presence of indomethacin [1 × 10-6]. The results showed that the estradiol derivative increased the LVP in a dependent dose manner and this effect was not inhibited in presence of indomethacin [1 × 10-6]. Each bar represents the mean ± S.E. of 9 experiments.
Alternative experimental indicate that effect induced by the estradiol derivative on the left ventricular pressure (Figure 7, see) in presence of tamoxifen at a concentration of 1 ×10-6 mmol wassignificantly blocked (p = 0.05).
Figure 7: Effects induced by the estradiol derivative on left ventricular pressure (LVP) through estrogen receptors. Intracoronary boluses (50 μl) of the estradiol derivative [1 × 10-9 to 1 × 10-4 mmol] were administered and the corresponding effect on the LVP was determined. The results showed that the estradiol derivative increased the LVP in a dependent dose manner and this effect was significantly inhibited (p = 0.05) in presence of tamoxifen [1 × 10-6]. Each bar represents the mean ± S.E. of 9 experiments.
Discussion
Synthesis chemical
Many procedures for the synthesis of aldehydes derivativesare available in the literature; for example, the synthesis of aldehyde 1,1-diacetates using morpholinium bisulfate as Bronsted acidic ionic liquid under solventfree conditions[23]. In addition, other reports indicate the synthesis of N- and side chain protected aspartyl and glutamyl aldehyde derivatives, using lithium tris(tertbutoxy) aluminium hydride or lithium tris[(3-ethyl-3- pentyl)oxy]aluminium hydride[24] as catalyst. Other data indicate the partial reduction of carboxylic acids with thexylchloroborane-methyl sulfideusing diisobutylaluminium hydrideas catalyst [25]. All these data indicate several methods for the synthesis of aldehyde derivatives; nevertheless,despite their wide scope, these procedures suffer fromseveral drawbacks; some reagents are of limited stability,and preparation can be dangerous. Therefore, in thiswork we report a straightforward route for synthesis of anew estradiol derivative using trichloromethane/ ethanol in basic medium. Thestructure of estradiol derivative was confirmed using IRand NMR spectroscopy. The IR spectra containedcharacteristic vibrations at 3380 for hydroxyl groups; at3300 for aldehyde groups.The 1H NMR spectrum of the estradiol derivative showssignals at 0.80-2.77, 3.44 and 7.84 ppm for steroidnucleus. In addition, other signals at 0.65 ppm for methylgroup; at 10.01 ppm for carbonyl group. Finally, the spectrum contains asignal at 10.53 ppm for both hydroxyl groups.The 13C NMR spectra displays chemical shifts at 23.95-168.01 ppm for steroid nucleus. In addition, other signals at 13.86 ppm for methyl group and at 192.83 ppm for carbon of aldehyde groups. In addition, the presence of brucine derivative was further confirmed from mass spectrum which showed a molecular ion at m/z 327.14.
Biological evaluation
First stage
The activity induced by an estradiol derivative on the perfusion pressure and coronary resistance in isolated rat heart (Langendorff technique) was evaluated. The results obtained showed that, the estradiol derivative significantly increased the perfusion pressure in comparison with the control conditions. Those experimental data indicate that, the estradiol derivative exerts effects on perfusion pressure, which could consequently bring modifications in coronary resistanceas happening in other type of steroid derivatives [26, 27]. In order to verify this hypothesis, the effects induced by estradiol on coronary resistance were evaluated. The results indicate that coronary resistance in presence of estradiol derivative was higher in comparison with control conditions. All this data suggest that estradiol derivative may induce a positive inotropic activity in the isolated rat heart.
Second stage
In the search of molecular mechanism involved in the inotropic activity of the estradiol derivative, some reports were analyzed. These reports indicate that estradiol exert an indirect tonic effect on adrenal catecholamines concentration[ 28, 29]. To evaluate this hypothesis and to characterize the molecular mechanism of this phenomenon, in this studythe effect exerted by the estradiol derivative on left ventricular pressure was evaluated in absence or presence of prazosin or metoprolol. The results showed that, the effect induced by the estradiol-derivative was not inhibited in presence of these compounds. These data indicate that the molecular mechanism involved in the effects of this steroid-derivative on left ventricular pressure is not through adrenergic activity. Therefore, analyzing these results and other reports which suggest that activity induced by an estradiol derivative on blood pressure involved a molecular mechanism via calcium-channels[14]. In this work, the activity induced by this steroid derivative on left ventricular pressure was evaluated in absence or presence of nifedipine. The results showed that effect exerted by estradiol derivative was not inhibited in presence of nifedipine. These results suggest that, the effect of the estradiol derivative on left ventricular pressure is notthroughL- type calcium channel.
In the search for other possible mechanisms involved in the inotropic activity exerted by the estradiol derivative and analyzing some reports which suggest that some steroid derivatives exert their effect on left ventricular pressure through via prostanglandin synthesis [30]. Therefore, in this study the activities induced by the estradiol derivative on left ventricular pressure were asses in absence or presence of indomethacin. The results indicate that effect exert by the estradiol derivative was not blocked by indomethacin, these data suggest that molecular mechanism involved in the activity of steroid derivative was not via prostanglandins. Finally, analyzing all these results and other reports[31], which indicate that some estradiol derivatives exert its effect by activation of estrogen receptor in the vascular smooth muscle.For this reason, we used tamoxifen an estrogen receptor blocker [15]to determine if the inotropic activity of estradiol derivative on left ventricular pressure was via the estrogen receptor which may be a key requirement for the biological activity. Our results showed that the effect exerted by the estradiol derivative was inhibited by tamoxifen, suggesting that the molecular mechanism is via the estrogen-receptor. Analyzing these data, it is important to mention that the estradiolderivative is a particularly interesting drug because the positive inotropic activity induced by this steroid derivative involves a molecular mechanism different in comparison with other inotropic drugs [32]. This phenomenon may result in a decrease in adverse effects such as cardiac arrhythmia and ischaemia induced by several cardiotonic agents such as cardiac glycosides and sympathomimetic amines [25].
Conclusions
All these experimental data suggest that, inotropic activitypositively induced byestradiolderivative on the leftventricular pressure may involve estrogen receptor activation.
References
- Braunwald E, Bristow M. Congestive Heart Failure: Fifty Years of Progress, Circulation. 2000; 102: 14-23.
- Feldman A. Classification of positive inotropic agents, J Am Coll Cardiol. 22 (1993)1223.
- Cohn J, Archibald D, Ziesche S, Franciosa J, Harston W, Tristani F, Dunkman W, Jacobs W, Francis G, Flohr K. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans dministration Cooperative Study. New Eng J Med. 1986; 314:1547-1552.
- Kersten J, Montgomery M, Pagel S, Warltier D. Levosimendan a New Positive Inotropic Drug, Decreases Myocardial Infarct Size via Activation of KATP Channels. Anesth Analg. 2000; 90: 5-11.
- Silverberg D, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, Brosh D. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000; 35: 1737-1744.
- Lederer W, Tsien R. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in purkinjefibres. J Gral Phsiol. 1976; 263: 73-100.
- Wier W, Hess P. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J Gral Phsiol. 1984; 83: 395-415.
- Clark AJ. The mode of action of strophantidin upon cardiac tissue. J Pharm Exp Tiss. 1914; 5: 215-234.
- Hart G, Noble D, Shimoni Y. The effects of low concentrations of cardiotonic steroids on membrane currents and tension in sheep purkinjefibres. J Physiol. 1983;334: 103-131.
- Pignier C, Keller M, Vié B, Vacher B, Santelli M, Niggli E, Egger M. A novel steroid-like compound F90927 exerting positive-inotropic effects in cardiac muscle. Br J Pharmacol. 2006; 147: 772-782.
- De Munari S, Cerri A, Gobbini M, Almirante N, Banfi L, Carzana G, Ferrari P. Structure-Based Design and Synthesis of Novel Potent Na+, K+-ATPase Inhibitors Derived from a 5,14-Androstane Scaffold as Positive Inotropic Compounds. J Med Chem. 2003; 46: 3644-3654.
- Gobbini M, Barassi P, Cerri A, De Munari S, Fedrizzi G, Santagostino M, Schiavone A. 17 alpha-O-(aminoalkyl) oxime derivatives of 3 beta, 14 betadihydroxy- 5 beta-androstane and 3 beta-hydroxy-14-oxoseco-D-5 beta-androstane as inhibitors of Na(+),K(+)-ATPase at the digitalis receptor. J Med Chem.2001; 44: 3821-3830.
- Templeton J, Kumar V, Cote D, Bose D, Elliott D, Kim R, LaBella F. Progesterone derivatives that bind to the digitalis receptor: synthesis of 14. betahydroxyprogesterone: a novel steroid with positive inotropic activity. J Med Chem. 1987; 30: 1502-1505.
- Figueroa-Valverde L Diaz-Cedillo F, Lopez-Ramos M,Garcia-Cervera E, Quijano K, Cordoba J. Changes induced by estradiol-ethylenediamine derivative on perfusion pressure and coronary resistance in isolated rat heart: L-type calcium channel. Biomed Pap. 2011; 155: 27-32.
- Shiau A, Barstad D, Loria P, Cheng L, Kushner P, Agard D, Greene G. The Structural Basis of Estrogen Receptor/Coactivator Recognition and the Antagonismof This Interaction by Tamoxifen. Cell. 1998; 95: 927-937.
- Graham R, Oates H, Stoker L, Stokes G. Alpha blocking action of the antihypertensive agent, prazosin. J Pharmacol Exper Ther. 1977; 201: 747-752.
- Bengtsson C, Johnsson G, Regårdh C. Plasma levels and effects of metoprolol on blood pressure and heart rate in hypertensive patients after an acute dose and between two doses during long-term treatment. Clin Pharmacol Ther. 1975; 17: 400-408.
- Henry P. Comparative pharmacology of calcium antagonists: nifedipine, verapamil and diltiazem. Am J Cardiol. 1980; 46: 1047-1058.
- Owen T, Ehrhart I, Weidner W, Scott J, Haddy F. Effects of indomethacin on local blood flow regulation in canine heart and kidney. Exp Biol Med. 1975; 149: 871-876.
- Bayne K. Revised Guide for the Care and Use of Laboratory Animals available. American Physiological Society. Physiologist. 1996; 39: 208-211.
- Figueroa-Valverde L, Díaz-Cedillo F, López-Ramos M, García-Cervera E, Quijano-Ascencio K, Cordova-Vazquez J. Synthesis of a new inotropic steroid derivative and its relationship with logP, Rm, Vm, Pt and ST. Asian J Chem. 2011; 23: 1599-1604.
- Hocht C, Opezzo L, Gorzalczany S, Bramuglia G, Tiara C. Una aproximación cinética y dinámica de metildopa en ratas con coartación aórtica mediante microdiálisis. Rev Argent Cardiol. 1999; 67: 769-773.
- Reza A, Nasreesfahani Z, Ruoho A. An efficient and chemoselective synthesis of aldehyde 1, 1-diacetates using morpholinium bisulfate as Bronsted acidic ionic liquid under solvent-free conditions. Organic reparations and Procedures International: New J Org Synt. 2008; 40: 385-391.
- Paris M, Pothion C, Heitz A, Martinez J, Fehrentz J.Synthesis of N- and side chain protected aspartyl and glutamyl aldehyde derivatives. Reinvestigation of the reduction of Weinreb amides. Tet Lett. 1998; 39: 1341-1342.
- Brown H, Soon J, Min N, Nazer B. Selective reductions. Partial reduction of carboxylic acids with thexylchloroborane- methyl sulfide. A direct and simple aldehyde synthesis. J Org Chem.1987; 52: 5400-5406.
- Ford S, Weber L, Stormshak F. Role of estradiol-17β and progesterone in regular constriction of ovine uterine arteries. Biol Reprod. 1977; 17: 480-483.
- Richard E, White, David J. Darkow, Jessica L, Lang F. Estrogen Relaxes Coronary Arteries by Opening BKCa Channels Through a cGMP-Dependent Mechanism. Circ Res.1995; 77: 936-942.
- Ma L, Robinson C, Thadani U, Patterson E. Effect of 17-beta estradiol in the rabbit: endothelium-dependent and independent mechanisms of vascular relaxation. J Cardiov Pharmacol. 1997; 30(1): 130-135.
- Ceballos G, Figueroa L, Rubio I, Garcia A, Martinez A, Yanez R. Acute and nongenomic effects of testosterone on isolated and perfused rat heart. J Cardiovasc Pharmacol. 1999; 33: 691-697.
- Figueroa-Valverde L, Diaz-Cedillo F, Diaz-Ku E, Camacho-Luis A. Effect induced by hemisuccinate of pregnenolone on perfusion pressure and vascular resistance in isolated rat heart. African J Pharm Pharmacol. 2009; 3: 234-241.
- Figueroa-Valverde L, Díaz-Cedillo F, López-Ramos M, García-Cervera E, Pool-Gómez E. Design and synthesis of an estradiol derivative and evaluation of its inotropic activity in isolated rat heart. African J Pharm Pharmacol. 2011; 5: 1638-1647.
- Colucci W, Gimbrone M, McLaughlin M, Halpern W, Wayne A. Increased Vascular Catecholamine Sensitivity and Adrenergic Receptor Affinity in Female and Estrogen Treated Male Rats. Circ Res. 1982; 50: 805-811.
- Lilley J, Golden J, Stone R. Adrenergic regulation of blood pressure in chronic renal failure. J Clin Invest.1976; 57: 1190-1200.
- Seillan C, Ody C, Russo F, Duval D. Differential aspects of sex steroids on prostaglandin secretion by male and female cultured piglet endothelial cells. Prostaglandins. 1983; 26: 3-12.
- Karas RH, Patterson BL, Mendelsohn M. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation. 1994; 89: 1943-1950.
- Akera T, Brody M. The role of Na+, K+-ATPase in the inotropic action of digitalis. Pharmacol. 1977; 29: 187.
- Pierard, Berthe C, Albert A, Carlier J, Kulbertus H. Haemodynamic alterations during ischaemia induced by dobutamine stress testing. Eur Heart J. 1989; 10: 783- 790.