ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 4
PLA2 inhibitory, antioxidant and antibacterial properties of various solvent extracts from R. frangula leaves
Moubayed NMS1 and Ben Bacha A2,3*
1Botany and Microbiology Department, Science College, King Saud University, Saudi Arabia
2Biochemistry Department, Science College, King Saud University, Saudi Arabia
3Laboratory of Plant Biotechnology Applied to Crop Improvement, Faculty of Science of Sfax, University of Sfax, Tunisia
- *Corresponding Author:
- Ben Bacha A
Department of Biochemistry
Science College, King Saud University, Saudi Arabia
Accepted on September 29, 2016
The total tannin, flavonoid and phenolic contents of R. frangula leaves were determined by colorimetric assay. The antioxidant and antibacterial activities of solvent extracts were investigated using various tests. The anti-PLA2 properties of these extracts were evaluated by measuring their inhibition potency on the human and dromedary pro-inflammatory phospholipase A2-IIA. The data showed that the water extract exhibited the highest amount of tannin compounds, whereas the total phenolic and flavonoid contents were highest in the methanol fraction. The same fraction was the most effective at the minimum inhibitory concentration against all strains tested and exhibited an antioxidant activity. In contrast, the water extract showed the best anti-PLA2-IIA activity, and no effect was recorded on the digestive DrGIB. The results indicated a strong correlation between the antioxidant capacity, the total phenolic and flavonoid contents. However, no correlation was observed with the inhibitory effect against PLA2, suggesting that the anti-PLA2 molecules were tannin compounds.
Keywords
R. frangula, Antioxidant, Antibacterial, Anti-PLA2.
Introduction
In recent years, increasing interest has been devoted to the research on and application of plants feed and food supplements and drugs [1]. Spices and herbs, which constitute essential parts of the human diet, are a safe and rich source of secondary biomolecules that exhibit significant pharmacological effects [2]. In fact, in addition to their use to improve the aroma, colour, and flavour of foods and in traditional medicine, spices and herbs also play antimicrobial [3], anti-oxidative [4], and preservative [5] roles.
Based on their ability to inhibit lipid peroxidation and scavenge free radicals, antioxidants extracted from plants play a main role in preventing diseases provoked by oxidative stress, like neurodegenerative ones, namely autism spectrum disorders [6] and Alzheimer’s disease [7]. Several reports have shown that plant extracts can be utilized as alternatives to antibiotics thanks to their beneficial effect on the animal intestinal tract besides their antimicrobial properties [8]. Craig [2] demonstrated that herbs and spices may strongly affect the function and reactivity of the farm animals’ immune system. Stability of feed and impact the digestive micro-population is highly enhanced by growth-promoting active feed supplements, essentially by inhibiting the growth of the pathogenic microorganisms. Considering the improved health state of the intestinal system, the farm animals are thus less exposed to the toxins produced by different microorganisms [1]. Windisch et al., [9] reported that herbs and spices amplify the absorption of essential nutrients and exhibit beneficial effects on the stress resistance of animals.
Secretory phospholipases A2 (sPLA2; EC 3.1.1.4) constitute a large family of small molecular mass proteins (14-19 kDa) with conserved structures and specifically catalyse the hydrolysis of fatty acid ester linkage to liberate lysophospholipids and free fatty acids [10]. Numerous sPLA2 have been characterized and classified into 12 different groups based on their structural features, of which group IIA sPLA2 (sPLA2-IIA) is the best known [11,12].
sPLA2-IIA plays a critical role in the initiation and amplification of inflammatory reactions [13]. Several families of eicosanoids (including leukotrienes and prostaglandins) produced from arachidonic acid catabolism by the lipooxygenase or cyclo-oxygenase pathways are involved in the inflammatory processes [14,15]. Thus, phospholipid metabolism control through sPLA2 inhibition presents potential value [16]. However, although several compounds have been suggested as inhibitors of different sPLA2s, no clinical investigations have attained a therapeutic phase. The present study aimed to examine the efficiency of several solvents to extract the main compounds from Rhamnus frangula (R. frangula) leaves and to evaluate the antioxidant, antimicrobial and PLA2 inhibitory activities of different extracts from R. frangula leaves.
Material and Methods
Preparation of crude extracts
The mature fresh leaves of R. frangula were collected from the Riyadh region of (Saudi Arabia), thoroughly washed in distilled water air-dried at room temperature and then grounded. 200 g of the obtained powder was extracted by maceration at room temperature for 72 h with ethanol. After that, the slurry was filtered through a Buchner funnel, and the obtained filtrate was centrifuged (15 min at 10,000 rpm and at 4°C), and lyophilized yielding the ethanolic extract. This fraction was then re suspended in water and partitioned successively with various solvents with different polarities (methanol, ethyl acetate, chloroform and butanol). All obtained fractions including the remaining solution which is designated “water extract” were stored at 4°C before analysis.
Total phenolic content
Folin-Ciocalteu’s reagent was used to determine the amount of total phenolics in the extracts from R. frangula leaves, as previously described by Velioglu et al., [17]. Briefly, a volume of 0.1 mL of each sample extract (1 mg/mL) was mixed with 0.75 mL of Folin-Ciocalteu reagent (previously diluted 10- times with deionized water) and incubated for 5 min at 25°C. Then, a volume of 0.75 mL of saturated sodium carbonate solution was added to the mixture. After incubation for 90 min at 25°C, the absorbance was read at 725 nm. The total phenolic compounds concentration was expressed in the different R. frangula extracts as mg gallic acid equivalents per gram of dry weight (mg GAE/g DW) using a calibration curve with gallic acid used as the reference standard ranging from 0 to 250 μg/mL (r2=0.99).
Total flavonoid content
The flavonoid content in the various extracts was determined by the aluminium chloride method based on the formation of a flavonoid-aluminium complex [18]. Briefly, a volume of 1.5 mL of extracts was mixed with equal volumes of 2% aluminium chloride hexahydrate solution, and the obtained mixture was vigorously shaken. After 10 min incubation, the absorbance at 367 nm was read. Using a standard curve of quercetin ranging from 0 to 50 μg/mL (r2=0.99), the total flavonoid content was calculated and expressed as mg quercetin/g dry weight (mg QE/g DW).
Tannin content
Tannin content was measured in each extract sample according to the vanillin protocol described by Sun et al., [19]. 50 μL of each suitably diluted sample was mixed with 1.5 mL of concentrated H2SO4 and 3 mL of a 4% methanol-vanillin solution and kept for 15 min at 4°C. Then, the absorbance was measured at 500 nm against methanol as a blank. Using a calibration curve of catechin, ranging from 0 to 400 μg/mL (r2=0.99), the amount of total condensed tannin was calculated and expressed as mg catechin/g dry weight (mg CE/g DW).
DPPH radical scavenging assay
The antioxidant activity of the R. frangula leaves fractions was measured using the 1,1 Diphenyl-2-Picrylhydrazyl (DPPH) radical scavenging method [20]. Briefly, a 0.5 mL volume of each sample concentration was mixed with an equal volume of DPPH ethanolic solution and shaken vigorously. After incubation in darkness for 1 h at room temperature, the absorbance of the residual DPPH radicals was determined at 519 nm and was compared to the control (containing all reagents except the R. frangula leaves extract). The scavenging of the DPPH radical was calculated as follows: Scavenging effect (%)=(1-ASample/AControl) × 100, where ASample and AControl are the absorbance of the sample and of the control, respectively. The extract concentration providing 50% inhibition (IC50) was calculated from the plot of the scavenging effect (%) versus the extract concentration.
Reducing power assay
The reducing power of the extracts from R. frangula leaves was investigated as previously described by Oyaizu [21]. Extracts with variable concentrations ranging from 0.03 to 1 mg/mL were mixed with 1 mL of 1% potassium ferricyanide and 1 mL of 0.2 M sodium phosphate buffer (pH 6.6). After incubation for 20 min at 50°C, 1.25 mL of 20% TCA was added to the obtained mixture and was centrifuged for 10 min at 3000 rpm. Finally, the upper layer solution (1.25 mL) was mixed with an equal volume of deionized water and 0.5 mL of 0.1% fresh ferric chloride. The absorption of the obtained mixture was measured at 700 nm using distilled water as the blank and BHT as a positive control.
Inhibition of PLA2 activity
The inhibitory effect of various extracts was assayed according to the method reported by De Aranjo and Radvany [22] using three secreted phospholipases: Human Group IIA phospholipase A2 (hG-IIA, 0.02 μg/μL), Dromedary Group IIA phospholipase A2 (DrG-IIA, 0.02 μg/μL) and Dromedary Group IB phospholipase A2 (DrG-IB, 0.002 μg/μL). Ten microliters of these sPLA2 solutions was mixed with 10 μL of each extract and the obtained mixture was incubated at room temperature for 20 min. Finally, 1 mL of the PLA2 substrate (3.5 mM lecithin suspended in 100 mM NaCl, 10 mM CaCl2, 3 mM NaTDC and 0.055 mM red phenol, pH 7.6) was added. The hydrolysis kinetics was followed spectrophotometrically at 558 nm for 5 min. The results are reported as the inhibition percentage that was calculated by comparison with a control experiment (absence of extract). The IC50 values were determined from the curve.
Antibacterial activity
The antibacterial activities of the different solvent extracts were determined by the agar diffusion method according to Berghe and Vlietinck [23] using several Gram-negative bacteria: Escherichia coli (ATCC 25966), Klebsiella pneumonia (ATCC 700603), Pseudomonas aeruginosa (ATCC 27853), Salmonella enteric (ATCC 43972) and Gram-positive bacteria: Bacillus cereus (ATCC 14579), Bacillus subtilis (ATCC 6633), Enterococcus faecalis (ATCC 29122), Staphylococcus aureus (ATCC 25923) and Staphylococcus epidermidis (ATCC 14990). The dried extracts were dissolved in 100% DMSO to a final concentration of 10 mg/mL and were filtered through a 0.22 mm Millipore filter. The bacterial strains were cultured for 24 h in a nutriment broth. Two hundred microliters of each bacterial suspension (106 CFU) was spread on Luria broth agar, and pores were then loaded with 10 μl of each sample extract. The plates were incubated overnight at 37°C. Ampicillin (10 μg/well) and DMSO were used as the positive reference standard and the negative control, respectively. The measurement of inhibition zones (in millimetres) on the surface of the top agar was performed three times, and the reported values are the averages of three separate assays.
Determination of the minimal inhibitory concentration (MIC)
The micro-well dilution method was used for the determination of the MIC values, which represent the lowest plant extract concentration that completely inhibits the growth of microorganisms [24]. Dilution series of each extract sample (dissolved in 100% DMSO) were prepared in a 96-well plate, ranging from 10 μg/mL to 5 mg/mL. Growth medium (40 μl), inoculums (10 μl) and diluted sample extract (50 μl) were mixed in each well and kept at 37°C for 24 h. After the addition of 40 μl of MTT (0.5 mg/mL) to each well, the mixture was incubated for 30 min. The MIC was considered as the well where no change to red colour of MTT was observed. DMSO and ampicillin were used as negative and positive controls, respectively. The MIC values were measured in triplicate.
Statistical analysis
Data are presented as the mean value ± SD of at least three replicates for each sample. Microsoft Excel software was used for the statistical analyses. p<0.05 were considered to be significant.
Results and Discussion
Extraction yields, total phenolic, flavonoid and tannin contents and antioxidant activity
R. frangula leaves were extracted using six solvents at different polarities (water, methanol, ethanol, ethyl acetate, chloroform and butanol). The extraction yields, as well as the phenolic, flavonoid and tannin contents, of the extractions from R. frangula leaves are summarized in Table 1.
| Extracts | Extraction yield (%) | Total phenolics (mg GAE/g DW) |
Total flavonoids (mg QE/g DW) |
Total tannins (mg CE/g DW) |
|---|---|---|---|---|
| Butanol | 17.4 ± 0.7c | 19.7 ± 1.1a | 1.85 ± 0.08c | 112 ± 3.5b |
| Chloroform | 42 ± 1.1e | 32.1 ± 1.6c | 1.94 ± 0.1b | 147 ± 4.1f |
| Ethanol | 100e | 56.7 ± 2.8d | 4.97 ± 0.09f | 127 ± 2.8d |
| Ethylacetate | 29.6 ± 1.7c | 23.6 ± 2.1e | 3.07 ± 0.1d | 135 ± 2.9e |
| Methanol | 59.2 ± 2.5a | 61.2 ± 3.1f | 5.07 ± 0.8c | 209 ± 4.1b |
| Water | 11.03 ± 0.8c | 9.05 ± 0.4c | 0.36 ± 0.04c | 289 ± 3.7c |
Table 1. Extraction yield and total phenolic, flavonoid and tannin contents of the extracts from R. frangula leaves.
The extraction yields with ethyl acetate, butanol and water showed the lowest amount of total extractable compounds, whereas the highest yields were observed with ethanol, methanol and chloroform (Table 1). This yield variation could be attributed to the polarities of the different compounds contained in R. frangula leaves.
Several reports demonstrated that phenolic molecules are the most potent antioxidant compounds [25,26] and have antimicrobial properties [27]. Therefore, the antioxidant potential of the various extracts from R. frangula leaves was estimated by determining their total phenolic, flavonoid and tannin contents and their free radical scavenging ability. The results given in Table 1 revealed that there are differences in the total flavonoid, phenolic and tannin contents of the different examined fractions. The amount of phenolic compounds varied from 9.05 ± 0.4 to 61 ± 3.1 mg GAE/g DW of extract. Indeed, the methanol extract showed the highest amount of phenolic compounds (61 ± 3.1 mg GAE/g DW), followed by the ethanol (56.7 ± 2.8 mg GAE/g DW), chloroform (32.1 ± 1.6 mg GAE/g DW), ethyl acetate (23.6 ± 2.1 mg GAE/g DW) and butanol (19.7 ± 1.1 mg GAE/g DW) extracts, whereas the poorest extract was the water extract (9.05 ± 0.04 mg GAE/g DW).
Table 1 also shows that the amount of flavonoid compounds ranged from 0.36 ± 0.04 to 5.07 ± 0.8 mg QE/g DW of extract. The methanol fraction recorded the highest content for flavonoids (5.07 ± 0.8 mg QE/g DW), followed by the ethanol (4.97 ± 0.09 mg QE/g D) and ethyl acetate (3.07 ± 0.1 mg QE/g D) fractions. The chloroform and butanol fractions had approximately the same values of total flavonoids (1.94 ± 0.1 and 1.85 ± 0.08 mg QE/g DW, respectively). The data clearly indicated that the highest content of flavonoids was obtained with a decrease in the polarity of the solvent used. In contrast, the water and methanol fractions displayed the highest tannins contents (289 ± 3.7 mg CE/g DW and 209 ± 4.1 mg CE/g DW, respectively) compared to the chloroform (147 ± 4.1 mg CE/g DW) and ethyl acetate fractions (135 ± 2.9 mg CE/g DW), and the ethanol and butanol fractions were the poorest (127 ± 2.8 mg CE/g DW and 112 ± 3.5 mg CE/g DW, respectively) (Table 1). The variations in the flavonoid, phenolic and tannin compounds of the various fractions could be explained by different polarities of the raw material compounds.
According to the literature, a single report has addressed the investigation of the content of main groups of chemical compounds (tannins, glucofrangulins, phenolic acids, flavonoids, total polyphenols and non-tannic polyphenols) in the bark of Frangula and Rhamnus species growing in Croatia [28]. The authors reported that all taxa studied contained elevated contents of total polyphenols (ranging from 2.68 to 8.50%); moderate contents of glucofrangulins (ranging from 0.22% to 09.26%), non-tannic polyphenols (ranging from 0.73% to 5.92%) and tannins (ranging from 1.10% to 4.92%); and low contents of phenolic acids (ranging from 0.44% to 1.81 %) and flavonoids (ranging from 0.02% to 1.44%), which are very low compared to our results. The present study confirmed that various solvent extracts from R. frangula leaves contain significant sources of phenolics, flavonoids and tannin antioxidants that may have therapeutic potential.
The in vitro free radical scavenging activity of R. frangula leaves extracts was tested through the DPPH method according to Tepe et al., [29]. The results are expressed as the mean of the IC50 values (mg/mL) and are presented in Table 2.
| Extracts | DPPH radical | Reducing power | |
|---|---|---|---|
| IC50 | Scavenging activity (%) at 1 mg/ml | EC50 (mg/ml)# | |
| (mg/ml)* | |||
| Butanol | 0.52 ± 0.02b | 32.5 ± 0.5 | 0.41 ± 0.02e |
| Chloroform | 0.21 ± 0.01e | 69.03 ± 0.65 | 0.19 ± 0.01c |
| Ethanol | 0.147 ± 0.03f | 85 ± 0.2 | 0.029 ± 0.002e |
| Ethyl acetate | 0.221 ± 0.05c | 41.17 ± 0.1 | 0.1 ± 0.03g |
| Methanol | 0.105 ± 0.01d | 87.65 ± 0.47 | 0.025 ± 0.001c |
| Water | 0.95 ± 0.02a | 25.1 ± 0.6 | 0.59 ± 0.02c |
| BHT | 0.052 ± 0.004b | 95.02 ± 0.05 | 0.019 ± 0.001d |
| *:IC50 (mg/mL): the concentration at which 50% is inhibited; #:EC50 (mg/mL): effective concentration at which the absorbance is 0.5. | |||
Table 2. Antioxidant activities of the different solvent extracts from R. frangula leaves tested with two methods: DPPH radical-scavenging and reducing power activity. IC50 values on DPPH were calculated from the plot of the scavenging effect against the extract concentration. BHT was used as the standard. Experiments were
Our results showed that the antioxidant capacity of the extracts decreased in the order of the methanol extract (IC50=0.105 ± 0.01 mg/mL), the ethanol extract (IC50=0.147 ± 0.03 mg/ mL)>the chloroform extract (IC50=0.21 ± 0.01 mg/mL), the ethyl acetate extract (IC50=0. 221 ± 0.05 mg/mL), the butanol extract (IC50=0.52 ± 0.02 mg/mL)>the water extract (IC50=0.95 ± 0.02 mg/mL) (Table 2).
The IC50 value of BHT, which was also investigated for the sake of comparison was 0.052 ± 0.04 mg/mL.
The in vitro antioxidant studies of the different fractions and the level of DPPH radical scavenging of different concentrations (0.03-1 mg/mL) of R. frangula leaves extracts were performed using BHT as the standard. The scavenging activity of all samples was concentration-dependent. As recorded in Table 2, the maximum activity of the control and the plant extracts at 1 mg/mL was BTH (95.02 ± 0.05%), the methanol fraction (87.65 ± 0.47%), the ethanol fraction (85.02 ± 0.2%), the chloroform fraction (69.03 ± 0.65%), the ethyl acetate fraction (41.17 ± 0.01%), the butanol fraction (32.5 ± 0.5%) and the water fraction (25.1 ± 0.6%).
A strong correlation was obtained between the contents of antioxidant components (total phenolic content, r2=0.906; total flavonoid content, r2=0.725) and the IC50 values of the DPPH radical-scavenging activity of the different extracts, suggesting a possible biological function in the prevention of lipid membrane oxidation. However, a weak correlation (r2=0.005) was found between the tannin content and the DPPH radicalscavenging activity (Table 3).
| Total phenolic content (mg GAE/g) | Total flavonoid content (mg QE/g) | Total tannin content (mg CE/g) |
|
|---|---|---|---|
| DPPH (IC50) | 0.906 | 0.725 | 0.005 |
| Reducing power (EC50) | 0.327 | 0.464 | 0.391 |
| Total flavonoid content (mg QE/g) | 0.914 | - | - |
| Total tannin content (mg CE/g) | 0.04 | - | - |
| IC50 value of hG-IIA inhibition | 0.001 | 0.005 | 0.2 |
| IC50 value of DrG-IIA inhibition | 0.001 | 0.007 | 0.32 |
| IC50 value of DrG-IB inhibition | 0.78 | 0.654 | 0.001 |
Table 3. Linear correlation coefficients (r2) for the relationships between the assays for the various extracts from R. frangula leaves.
Fe3+-Fe2+ transformation in the presence of various R. frangula leaves extracts and BHT, a widely used commercial antioxidant used as the reference, was carried out to determine the reductive capability, which may serve as a strong indicator of the potential antioxidant activity. Throughout the concentration range (0-1 mg/mL), the methanol (EC50=0.025 ± 0.001 mg/mL) and ethanol (EC50=0.029 ± 0.002 mg/mL) fractions and the standard (EC50=0.019 ± 0.001 mg/mL) showed nearly the same tendency in their reducing power, though all the studied extracts were less effective than the standard. Ethyl acetate (EC50=0.1 ± 0.03 mg/mL) and chloroform (EC50=0.19 ± 0.01 mg/mL) extracts were better radical reducers compared to the butanol (EC50=0.41 ± 0.02 mg/mL) and water (EC50=0.59 ± 0.02 mg/mL) extracts (Table 2). The results indicate significant variations (p<0.05) among the examined extracts in the reducing power activity where the antioxidant activity was moderately correlated with the total phenolic (r2=0.327), tannin (r2=0.391) and total flavonoid (r2=0.464) contents (Table 3). The results are in agreement with several previous reports, which demonstrated a significant relationship between the total phenolic contents in plant extracts and their antioxidant power [17,30].
Evaluation of PLA2 inhibitory effect
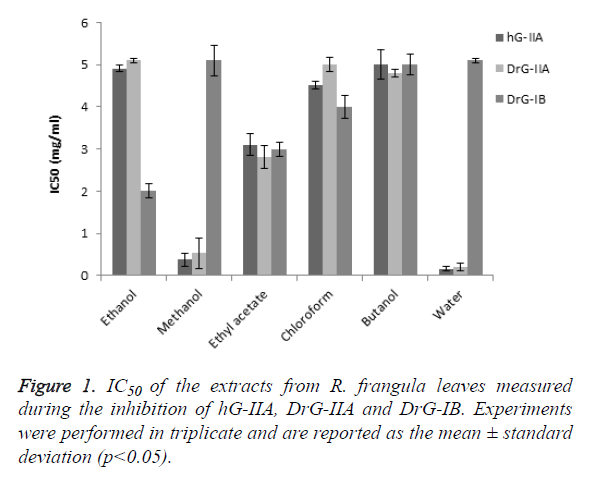
To assess the potential anti-inflammatory activity of R. frangula leaves, preliminary experiments searching for the PLA2 inhibitory activity of various extracts were performed using threesPLA2s: DrG-IB, which catalyses the hydrolysis of dietary phospholipids and hG-IIA and DrG-IIA, involved in the process of inflammation. These experiments aimed to select an extract that had no or minimal inhibitory effects on the digestive sPLA2-IB but it was capable to selectively inhibit the pro-inflammatory sPLA2-IIA. Figure 1 shows that out of the 6 extracts screened, four extracts (ethanol, methanol, ethyl-acetate and water) showed significant results (Figure 1). The water extract showed the most promising results in inhibiting the catalytic activity of both human and dromedary sPLA2-IIA, with an IC50 of 0.19 and 0.16 mg/mL, respectively. Interestingly, even at concentrations higher than 5 mg/mL, no inhibition of the sPLA2-IB activity was recorded for the same extract which indicates a selective inhibition of the water extract against both groups of sPLA2. On the other hand, a significant correlation between the IC50 measured during DrG-IB inhibition and the total flavonoid (r2=0.654) and phenolic (r2=0.78) compounds was also observed, whereas a very weak correlation was recorded with tannins (r2=0.001).The IC50 measured during sPLA2-IIA was only moderately correlated with tannins (hG-IIA: r2=0.2; DrG-IIA: r2=0.32). These results can be explained by the fact that the inhibition of these two groups of sPLA2 is due to different compounds. Furthermore, the inhibition of sPLA2-IIA activity but not that of DrGIB could be attributed to tannins which are present in significant amount in the water extract. Previous studies have suggested that the phenolic molecules could not be the compounds responsible for the anti-inflammatory effect [30-33].
Antibacterial activity
The antibacterial activity was checked against Gram-negative, Escherichia coli (ATCC 25966), Pseudomonas aeruginosa (ATCC 27853), Klebsiella pneumonia (ATCC 700603), Salmonella enteric (ATCC 43972), and Gram-positive, Staphylococcus epidermidis (ATCC 14990), Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29122), Bacillus cereus (ATCC 14579) and Bacillus subtilis (ATCC 6633), strains by measuring the inhibition zone diameter after the inoculation of bacteria with the solvent extracts in Luria broth agar media and MIC values determination (Table 4).
| Bacteria Strain | Inhibition zone (mm) | MIC (mg/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol | Methanol | Chloroform | Ethyl acetate | Butanol | Water | Methanol | Ethanol | Chloroform | Ethyl acetate | Butanol | Water | |
| Gram+ | - | |||||||||||
| B. cereus(ATCC 14579) | 20 ± 0.7b | 22 ± 0.5c | 15 ± 1.3a | 17 ± 0.8a | 11 ± 1c | 9 ± 0.8e | 78b | 156.25b | >1250 | >1250 | >2500 | - |
| B. subtilis(ATCC 6633). | 21 ± 0.5c | 24 ± 1.5c | 13 ± 0.9d | 15 ± 0.6a | 12 ± 0.9a | 5 ± 0.1b | 156.25c | >312.5 | >1250 | >1250 | >1250 | - |
| E. faecalis (ATCC 29122) | 17 ± 1.1b | 17 ± 1.1b | 10 ± 0.5b | 12 ± 1.1b | 7 ± 0.3b | 6 ± 0.2b | >625 | >625 | >1250 | >2500 | >2500 | - |
| S. epidermidis(ATCC 14990) | 17 ± 0.8d | 20 ± 0.6a | 16 ± 1.3b | 18 ± 1.6b | 12 ± 0.8b | 7 ± 0.2d | >156.25 | >156.25 | >625 | >1250 | >1250 | - |
| S. aureus (ATCC 25923) | 18 ± 1.5a | 21 ± 0.5b | 14 ± 0.8a | 16 ± 0.9c | 8 ± 0.2c | 8 ± 0.6c | 78a | >156.25 | >1250 | >625 | >2500 | - |
| Gram- | >1250 | >2500 | >2500 | - | ||||||||
| E. coli (ATCC 25966) | 10 ± 0.2b | 13 ± 0.7b | 7 ± 0.5d | 9 ± 0.5b | 4 ± 0.1d | - | >625 | >1250 | >1250 | >2500 | >2500 | - |
| K. pneumonia(ATCC 700603) | 11 ± 0.1b | 12 ± 1.1b | 5 ± 0.2b | 6 ± 0.2a | 5 ± 0.1b | - | >1250 | >1250 | >2500 | >2500 | >2500 | - |
| P. aeruginosa(ATCC 27853) | 10 ± 0.6b | 8 ± 0.2b | 6 ± 0.1a | 7 ± 0.3a | 2 ± 0.05b | - | >1250 | >1250 | >2500 | >2500 | >2500 | - |
| S. enteric (ATCC 43972) | 12 ± 0.8b | 15 ± 0.5b | 3 ± 0.05a | 8 ± 0.7b | 3 ± 0.05e | - | >312.5 | >625 | >1250 | >2500 | >2500 | - |
| The different letters (a-e) indicate a significant difference between the extracts at p<0.05. (-): Insensitivity. | ||||||||||||
Table 4. Antibacterial activity of the extracts from R. Frangula leaves on nine Gram-positive and Gram-negative bacteria.
Table 4 shows that all tested extracts had fluctuating degrees of antibacterial potency against the microorganisms examined, except for the water extract, which had neither bacteriostatic nor bactericidal effect against Gram-negative bacteria. The methanol extract was the most effective and exhibited a large antimicrobial spectrum and displayed potent antibacterial effect against all examined bacteria. The order of sensitivity to the methanol extract was Bacillus cereus>Bacillus subtilis>Staphylococcus aureus>Staphylococcus epidermis>Enterococcus faecalis>Salmonella enteric>Escherichia coli>Klebsiella pneumonia>Pseudomonas aeruginosa. Staphylococcus aureus and Bacillus cereus were the most sensitive microorganisms, with an MIC value of approximately 78 mg/mL, followed by Bacillus subtilis and Staphylococcus epidermis. These results are of great importance, particularly in the case of Staphylococcus aureus and Bacillus subtilis, which are resistant towards some antibiotics and produce several enterotoxins that provoke septicemia and enteritis [34]. The antibacterial activity of the methanol extract from R. frangula leaves could be related in particular to the presence of a high amount of tannins, in addition to the phenolics and flavonoids components. Tannins act as active detoxifying agents by precipitating the bacterial cell wall proteins and inhibiting bacterial growth [27].
Conclusion
The present study reported the total phenolic, flavonoid and tannin contents and the antioxidant, antibacterial and anti- PLA2 activities of various solvent extracts of R. frangula leaves. A strong correlation was found between the total flavonoid and phenolic contents and the antioxidant capability whereas no correlation was established with the anti-PLA2, suggesting that tannins molecules could be responsible for the anti-PLA2 effect. Furthermore, R. frangula leaves contain some major bioactive compounds that inhibit the growth of several microorganisms, thereby proving to be effective as an alternative source of antibiotics.
Acknowledgements
TThe Authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP-VPP-086
References
- Franki? T, Volj? M, Salobir J, Rezar V. Use of herbs and spices and their extracts in animal nutrition. Acta agriculturae Slovenica 2009; 94: 95-102.
- Craig JW. Health promoting properties of common herbs. A J Clini Nutri 1999; 70: 491S- 499S.
- Salie F, Eagles PFK, Leng HMJ. Preliminary antimicrobial screening of four South African Asteraceae species. J Ethno pharmacol 1996; 52: 27-33.
- Shobana S, Naidu KA. Antioxidant activity of selected Indian spices Prostaglandins Leukotienes and Essential Fatty Acids 2000; 62: 107-110.
- Neilsen PV, Rios R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int J Food Mic 2000; 60: 219-229.
- Kern JK, Haley BE, Geier DA, Sykes LK, King PG, Geier MR. Thimerosal Exposure and the Role of Sulfation Chemistry and Thiol Availability in Autism. J Environ Res Public Health 2013; 10: 3771-3800.
- Natarajan S, Shanmugiahthevar KP, Kasi PD. Cholinesterase inhibitors from Sargassum and Gracilaria gracilis: seaweeds inhabiting South Indian coastal areas (Hare Island Gulf of Mannar). Nat Prod Res 2009; 23: 355-369.
- Al-Kassien GAM. Influence of two plants extracts derived from thyme and cinnamon of broiler performance. Pakistan Vet J 2009; 29: 169- 173.
- Windisch W, Schedle K, Plitzner C, Kroismayer A. Use of phytogenetic products as food additives for swine and poultry. J Animal Sci 2008; 86: E140-E148.
- Kini RM. Bee Venom phospholipase A2, enzyme: structure, function and mechanism. Kini R.M., (ed.) Wiley, New York 1997; pp.1-26.
- Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 2011; 111: 6130-6185.
- Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta 2000; 1488: 1-19.
- Birts CN, Barton CH, Wilton DC. Catalytic and non-catalytic functions of human IIA phospholipase A2.Trends in Biochem Sci 2010; 35: 28-35.
- Rola-Pleszeynski M, Thivierge M, Gagnon N, Lacasse C, Stankova J. Differential regulation of cytokine and cytokine receptor genes by PAF, LTB4 and PGE2J. Lipid Mediators 1993; 6: 175-181.
- Pruzanski W, Vadas P. Phospholipase A2-a mediator between proximal and distal effectors of inflammation. Immunol Today 1991; 12: 143-146.
- Tibes U, Friebe WG. Phospholipase A2 inhibitors in development. Exp opin on invest drugs 1997; 6: 279-298.
- Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agri Food Chem 1998; 46: 4113-4117.
- Lamaison JLC, Carnet A. Teneurs en principaux flavonoids des fleurs de Crataegeusmonogyna Jacq et de Crataegeus laevigata (Poiret D. C) en fonction de la vegetation. Pharm Acta Helv 1990; 65: 315-320.
- Sun B, Richardo-da-Silvia JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. Agric Food Chem 1998; 46: 4267-4274.
- Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine glucose model system. I: investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J Oil Chemists’ Society 1998; 75: 181-187.
- Oyaizu M. Studies on products of the browning reaction prepared from glucose amine. Jpn J Nutr 1986; 44: 307-315.
- De Araújo AL, Radvanyi F. Determination of phospholipase A2 activity by a colorimetric assay using a pH indicator. Toxicon 1987; 25: 1181-1188.
- Berghe VA, Vlietinck AJ. Screening methods for anti-bacterial and antiviral agents from higher plants. Methods in Plant Biochem 1991; 6: 47-68.
- Wade D, Silveira A, Rollins-Smith L, Bergman T, Silberring J, Lankinen H. Hematological and antifungal properties of temporin A and a cecropin A-temporin A hybrid. Acta Biochim Polon 2001; 48: 1185-1189.
- Ksouri R, Megdiche W, Falleh H, Trabelsi N, Boulaaba M, Smaoui I, Abdelly A. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C R Biol 2008; 331: 865-873.
- Maisuthisakul P, Suttajit M, Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem 2007; 4: 1409-1418.
- Stevenson DE, Hurst RD. Polyphenolic phytochemicals-just antioxidants or much more? Cell Mol Life Sci 2007; 64: 2900-2916.
- Maleš Z, Kremer D, Randi? Z G, Randi? M, Pilepi? K H, Boji? M. Quantitative analysis of glucofrangulins and phenolic compounds in Croatian Rhamnus and frangula species. Acta Biologica Cracoviensia Series Botanica 2010; 52: 108-113.
- Tepe B, Daferera D, Sokmen A, Sokmen M, Polissiou M. Antimicrobial and antioxidant activities of essential oil and various extracts of Salviatomentosa miller (Lamiaceae). Food Chem 2005; 90: 333-340.
- Kammoun M, Miladi S, Ben Ali Y, Damak M, Gargouri Y, Bezzine S. In vitro study of the PLA2 inhibition and antioxidant activities of Aloe vera leaf skin extracts. Lip in Hlth and Dis 2011; 10: 30.
- Yagi A, Satoshi T. Anti-inflammatory constituents, Aloesin and Aloemannan in Aloe Species and effects of Tanshinon VI in Salvia miltiorrhiza on Heart Yakugaku zasshi. J of the Pharmceut soc of Jpn 2003; 123: 517-532.
- Esua MF, Rauwald JW. Novel bioactive maloyl glucans from Aloe vera gel: isolation, structure elucidation and in vitro bioassays. Carb Res 2006; 341: 355-364.
- Parc MY, Kwon HJ, Sung MK. Evaluation of Aloin and Aloe-Emodin as anti-inflammatory agents in Aloe by using murine macrophages. Biosc Biotech Biochem 2009; 73: 828-932.
- Hajji M, Jarraya R, Lassoued I, Masmoudi O, Damak M, Nasri M. GC/MS and LC/MS analysis and antioxidant and antimicrobial activities of various solvent extract from Mirabilis Jalapa tubers. Process Biochem 2010; 45: 1486-1493.