ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 21
L-NMMA delays apoptosis in the nucleus pulposus cells of intervertebral discs
Chun-Lei Liu1,2, Xiang-Jiang Wang2, Yi-He Hu1*, Bei-Ji Lu2 and Gui-Qing Wang2
1Department of Orthopedics, Xiangya Hospital, Central South University, Changsha, Hunan, P. R. China
2Department of Orthopedics, Qingyuan People’s Hospital, Qingyuan, Guangdong, P. R. China
- *Corresponding Author:
- Yi-He Hu
Department of Orthopedics
Xiangya Hospital, Central South University
Changsha, Hunan, P. R. China
Accepted date: September 07, 2017
Apoptosis plays an important role in degeneration of Nucleus Pulposus (NP) cells. L-Monomethyl- Arginine (L-NMMA) is an analogue of L-arginine and can be inactivated irreversibly by induced NOS (iNOS). L-NMMA can also compete with L-arginine for binding sites on iNOS molecules. In addition, LNMMA has been reported to influence the apoptosis of hyaline cartilage cells in vitro. Thus, we hypothesized that L-NMMA would influence the apoptosis of NP cells in vitro. Rabbit primary NP cells were cultured and subjected to flow cytometry to determine the levels of apoptosis in each group at each time point. Western blotting was performed to detect the Bcl-2/Bax ratio, as well as protein levels of caspase-3 and caspase-9. A significant increase in apoptosis was observed between the NP cells cultured with 1% FBS medium with versus without L-NMMA (P<0.01). Likewise, the Bcl-2/Bax ratio was also increased in NP cells cultured in 1% FBS with L-NMMA, versus those cultured without L-NMMA (P<0.05). Meanwhile, the activity levels of both caspase-3 and caspase-9 were reduced in the presence of L-NMMA, compared with levels observed in the absence of L-NMMA (P<0.01). This study showed that L-NMMA treatment delayed the senescence and apoptosis of NP cells in vitro, raising the possibility that L-NMMA may be a novel treatment to delay intervertebral disc degeneration.
Keywords
L-monomethyl-arginine, Nucleus pulposus cells, Apoptosis, Caspase-3, Caspase-9, Bcl-2, Bax
Introduction
The main clinical manifestation of degenerative disc disease includes severe pain in the back, neck, and shoulder. This pain has a relatively large impact on human spine function and can negatively affect both bones and organs [1]. To date, the exact pathogenesis of intervertebral disc degeneration remains elusive. Accumulating evidence suggests that Nitric Oxide (NO) may play a physiological role in neuronal signal transmission, and it is also involved in various disease processes [2,3]. Nitric Oxide (NO) was initially discovered as an endothelium-derived relaxing factor that contributes to regulating vascular tone. NO is formed from the amino acid precursor, L-arginine, by Nitric Oxide Synthase (NOS) [4]. Elevated NO production has been shown to play an important role in the pathogenesis of neural diseases such as Alzheimer’s, and excess levels of NO have been detected in the Cerebrospinal Fluid (CSF) of patients with degenerative lumbar disease compared with pain-free control patients [4-6].
The Nucleus Pulposus (NP) performs several critical functions, one of which is ensuring that compressive loads are evenly distributed and transferred between the vertebral bodies of intervertebral discs [7]. Intervertebral disc degeneration is characterized by a cascade of cellular, biochemical, and structural changes. This cascade begins in the central NP where decreasing proteoglycan content and an associated reduction in hydrostatic pressure impair the function of the NP, thereby leading to back pain [8,9]. NP cells only represent approximately 1% of the entire intervertebral disc tissue, yet they play an essential role in resisting mechanical loadings by synthesizing an Extracellular Matrix (ECM) to maintain the stability of intervertebral discs. The synthesized ECM also mediates an important protective effect on disc integrity [10,11]. Correspondingly, the loss of NP cells has been reported to closely correlate with the onset of intervertebral disc degeneration [12].
Numerous studies have revealed that NP cells may be induced to produce NO in response to certain stimuli [13,14]. In a study of chondrocytes by Maneiro et al., NO was found to activate mitochondria-dependent events and to induce mitochondrial pathways involved in mediating apoptosis [15]. For NP cells, recent studies have demonstrated that apoptosis plays an important role in their degeneration [16,17]. L-Monomethyl- Arginine (L-NMMA) is an analogue of L-arginine and can irreversibly inactivate induced NOS (iNOS) by competing with L-arginine for binding sites on iNOS molecules. This inactivation results in significantly lower levels of NO production and an inhibition of apoptosis. We hypothesize that NO-mediated apoptosis plays a critical role in the loss of NP cells in intervertebral disc degeneration. To test this hypothesis, primary NP cells were isolated and NO production was inhibited by L-NMMA to investigate the effects of NO on serum-induced apoptosis in NP cells cultured in vitro, and to study the mechanism(s) involved.
Materials and Methods
Equipment and reagents
A Nikon inverted microscope (Japan Nikon Corporation); an EPICS® ALTRATM flow cytometer (Beckman Coulter, Inc.); a 725S-visible spectrophotometer (Prism Optical Technology Co., Ltd., Shanghai, China); an Electrophoresis JY-300C unit (Beijing Liuyi Instrument Plant); DMEM medium, an anticollagen II antibody, ALP (C-8), and an immunocytochemistry kit (including goat anti-mouse and goat anti-rabbit secondary antibodies) (Ye Lei Biotechnology Co., Ltd., Guangzhou, China) were used. An Annexin V-FITC PI apoptosis detection kit, a Caspase-3 Detection Kit and a Caspase-9 Detection Kit were all purchased from Pik-day Biotechnology Institute (-). LNMMA was purchased from Sigma (St. Louis, MO, USA).
Isolation and culturing of NP cells
Twelve New Zealand white rabbits, independent of gender (weight 3 ± 0.5 kg), were anesthetized and sacrificed for the resection of lumber discs 1 through 4 under sterile conditions. The NP of each disc was removed and rinsed in D-Hank’s solution. Then the NP tissues were cut into small pieces with ophthalmology scissors and were digested with trypsin (0.25%) for 15 min. The resulting cell suspension was washed with Dhank’s solution, and then was re-suspended in 2 ml crude collagenase medium and DMEM containing Fetal Bovine Serum (FBS) (20%). After a centrifugation step to pellet the cells, the collagenase medium was removed and the cells were carefully washed with D-Hank’s solution. After a final centrifugation step, the cells were re-suspended in DMEM in flasks at a concentration of 2 × 104 cells/cm2. The cells were cultured to obtain a confluent monolayer before establishing the experimental treatment groups. To characterize the cells obtained, staining with toluidine blue and immunocytochemistry (ICC) to detect type II collagen were performed. Regarding the latter, an anti-collagen II antibody, ALP (C-8) was used in conjunction with goat anti-mouse and goat anti-rabbit secondary antibodies (Ye Lei Biotechnology Co., Ltd., Guangzhou, China). This study was approved by the Central South University Institutional Review Board (no. 201512136).
Experimental cell groups
After a monolayer culture of the isolated NP cells was established, NP cells from this primary culture were divided into three experimental groups: 10% FBS (cultured in medium with 10% FBS); 1% FBS (cultured in medium with 1% FBS); and 1%FBS+L-NMMA (cultured in medium with 1% FBS and 50 μM L-NMMA). These three treatments groups were cultured for 24 h, 48 h, and 72 h.
Characterization of apoptosis and caspase activity
An Annexin V-FITC PI apoptosis detection kit, a Caspase-3 Detection Kit, and a Caspase-9 Detection Kit were all purchased from Pik-day Biotechnology Institute (Beyotime Institute of Biotechnology) and were used according to the manufacturer’s instructions.
Immunoblot detection of Bcl-2 and Bax
Expression levels of Bcl-2 and Bax expression were detected by Western blotting. Briefly, NP cells were lysed with a detergent buffer containing: 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 0.02% sodium azide, 100 μg/ml PMSF, 1 μg/ml aprotinin, and 1% Triton X-100 (or NP-40). The total protein concentration of each sample was determined by using the Bradford method. Western blotting was performed using Bcl-2 and Bax primary antibodies (5 mg/1 mL; Ye Lei Biotechnology Co., Ltd., Guangzhou, China). Detection of GAPDH was performed as a loading control. A horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody was diluted 1:5000 as the secondary antibody (Ye Lei Biotechnology Co., Ltd.). Bound antibodies were detected using enhanced chemiluminescence reagents (Ye Lei Biotechnology Co., Ltd.). Image analysis software was used to analyze band densities (Tian Neng Biotechnology Co., Ltd., Shanghai, China).
Statistical methods
Values represent means ± standard errors of 3-5 trials involving independent cell isolations from separate samples. Analyses of variance (ANOVAs) were followed up with Student-Newman- Keuls pair-wise post hoc tests [18-20]. For small sample sizes, confidence intervals were calculated based on t-distribution. All data analyses were conducted in SPSS 17.0 software (IBM Incorporated, Armonk, NY). P-values less than 0.05 and less than 0.01 were considered significant and very significant, respectively.
Results
Morphology of NP cells
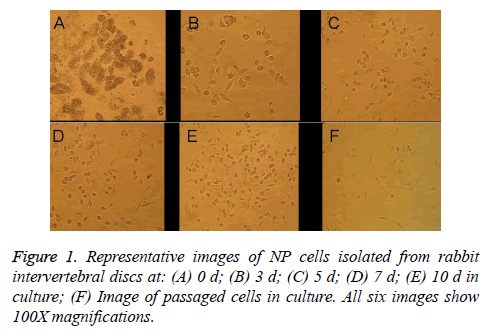
Primary NP cells (monolayer primary cultures established with cells isolated from rabbit intervertebral discs) had a small, round morphology, exhibited strong refraction, and were slow to affix to the container wall when suspended in culture medium (Figure 1A). After 2-3 d in culture, the cells were predominantly elliptical or small and round in shape with large nuclei and small cell volumes; they were scattered or distributed in sheets, exhibited some refraction, and had reached 20-30% confluence (Figure 1B). After 5-7 d in culture, cell confluence had increased to about 40% and a majority of the cells were fusiform in morphology with a protruding appearance, a round central nucleus, and one or two nucleoli, though some cells remained round or oval (Figures 1C and 1D). After the cells had been in culture for 10 d, cell confluence had reached 60-70%, the cells had abundant cytoplasm, and they were mainly triangular, spindled, or small and round in shape (Figure 1E), which indicated that postpassage cell morphology could be used in downstream tests. After passage, the cells grew rapidly and exhibited complete adherence much faster (~3d) than the original-generation cells, but they had intracellular vacuoles. In the second generation after passage, signs of aging were apparent, with ~80% of the cells taking on long shuttle morphology (Figure 1F).
Identification of NP cells
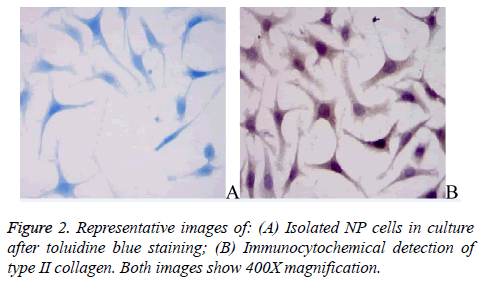
The primary NP cells exhibited strong toluidine blue staining near their nuclei (Figure 2A), consistent with the ability of NP cells to secrete proteoglycans. The NP cells were also immunopositive for type II collagen; the type II collagen staining was rather diffuse with stronger staining around nuclei (Figure 2B).
L-NMMA reduced apoptosis of NP cells
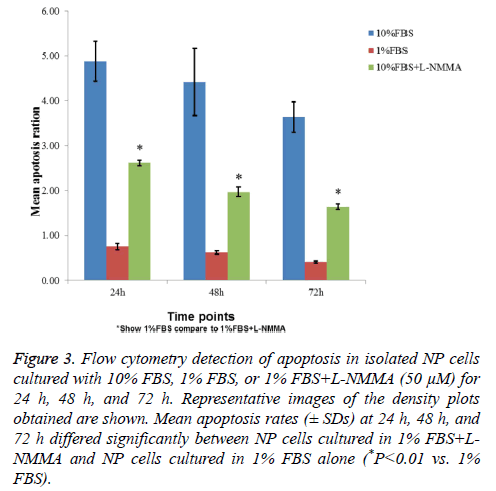
Flow cytometry showed that NP cells cultured with 1% FBS exhibited significantly higher levels of apoptosis than cells cultured with 10% FBS after 24 h, 48 h, and 72 h (Figure 3). Inclusion of 50 μM L-NMMA in NP cell cultures containing 1% FBS resulted in a significant reduction in cell death relative to 1% FBS (only) cultures (P<0.01, all three time points; Figure 3).
Figure 3: Flow cytometry detection of apoptosis in isolated NP cells cultured with 10% FBS, 1% FBS, or 1% FBS+L-NMMA (50 μM) for 24 h, 48 h, and 72 h. Representative images of the density plots obtained are shown. Mean apoptosis rates (± SDs) at 24 h, 48 h, and 72 h differed significantly between NP cells cultured in 1% FBS+LNMMA and NP cells cultured in 1% FBS alone (*P<0.01 vs. 1% FBS).
L-NMMA increased Bcl-2: Bax ratio in NP cells
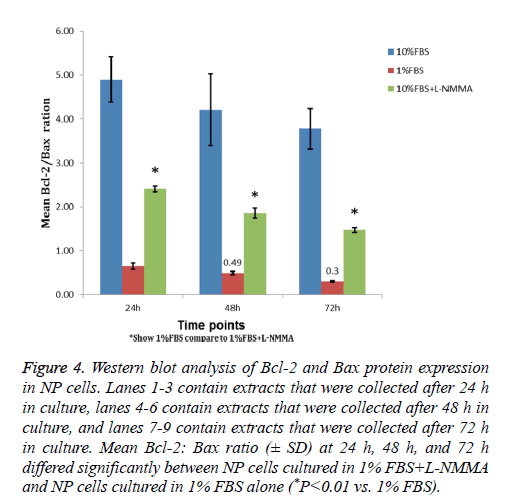
Western blot analysis showed that, among the three NP cell culture groups, the 1% FBS and 10% groups had the lowest and highest Bcl-2: Bax ratios (Figure 4). In addition, a significant increase in the Bcl-2: Bax ratio was detected for NP cells cultured in 1% FBS containing 50 μM L-NMMA compared to that of cells cultured in 1% FBS alone (P<0.05). The increased ratio reflected an increased level of Bcl-2 expression in NP cells cultured in 1% FBS containing 50 μM L-NMMA that corresponded with the significant decline in cell death described above for the same experimental group of cells.
Figure 4: Western blot analysis of Bcl-2 and Bax protein expression in NP cells. Lanes 1-3 contain extracts that were collected after 24 h in culture, lanes 4-6 contain extracts that were collected after 48 h in culture, and lanes 7-9 contain extracts that were collected after 72 h in culture. Mean Bcl-2: Bax ratio (± SD) at 24 h, 48 h, and 72 h differed significantly between NP cells cultured in 1% FBS+L-NMMA and NP cells cultured in 1% FBS alone (*P<0.01 vs. 1% FBS).
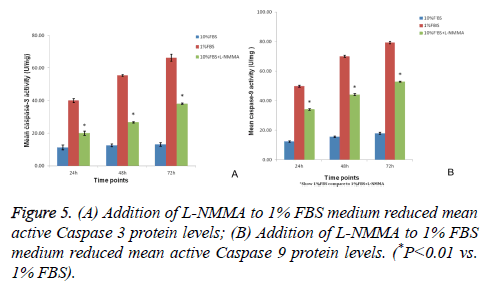
L-NMMA decreased caspase-3 and caspase-9 activity levels in NP cells
Caspase-3 and caspase-9 activity levels per mg of caspase protein in NP cells cultured in 1% FBS were significantly greater than the levels observed for NP cells cultured in 10% FBS after 24 h, 48 h, and 72 h of culturing (P<0.01). However, the activity levels of both caspase-3 and caspase-9 were significantly decreased at each time point when 50 μM LNMMA was added to 1% FBS cultures (all P<0.01; Figures 5A and 5B).
Discussion
Apoptosis is a normal physiological process that can be used to remove harmful or severely damaged cells. However, when this process becomes excessive, it represents a pathological phenomenon. Currently, the exact pathogenesis of intervertebral disc degeneration remains elusive, although the loss of NP cells due to apoptosis closely correlates with the onset on intervertebral disc degeneration and it has been shown to play an important role [18-20]. Therefore, over the past few years, the role of apoptosis in intervertebral disc degeneration has become an active area of research, and inhibition of apoptosis in NP cells may be an important strategy for delaying intervertebral disc degeneration.
Compared with other in vitro cell cultures, the culturing of NP cells is relatively difficult. Existing disc cell culture methods include organized block culture and enzymatic digestion [21-23]. For the former method, a longer adherent time is needed and the obtained cell number is limited. Cells have been isolated more thoroughly with enzyme digestion, and this method was successfully applied to the isolation and culturing of NP cells in the present study. Phenotypically, cartilage cells and NP cells are very similar and they both secrete proteoglycans and type II collagen [24,25]. Therefore, in the present study, ICC detection of type II collagen and staining of proteoglycans were performed to determine the purity of the primary NP cells that were isolated. Positive staining of the cultured NP cells with toluidine blue revealed that the isolated cells were metachromatic and they had the capacity to synthesize proteoglycans [26,27]. Detection of type II collagen further confirmed that the cultured cells were NP cells [26,27].
After obtaining the isolated NP cells, L-NMMA was administered to investigate apoptosis induced by serum starvation. L-NMMA is a congener of L-arginine and has been used as an inhibitor of NO production based on its ability to compete with NOS for L-arginine. The present results show that L-NMMA can rescue serum starvation-induced apoptosis in NP cells and NO plays an important role in the apoptosis of NP cells.
The Bax gene, located in human chromosome 19, is one of the most important genes in the Bcl-2 gene family and it promotes apoptosis. Bax and Bcl-2 can form heterodimers or homodimers, and only the combination of Bcl-2 and Bax mediates anti-apoptotic effects. Furthermore, it has been confirmed that the Bcl-2/Bax ratio negatively correlates with apoptosis [28-30]. In the present study, L-NMMA treatment of serum starved NP cells led to a significant rescue of the decrease in the Bcl-2/Bax ratio due to serum starvation, thereby confirming that NO is involved in mediating apoptosis in NP cells, and it mediates this effect, in part, by regulating the Bcl-2/Bax ratio.
Caspases contribute to a variety of important biological functions during apoptosis, including the initiation, regulation, and enforcement of this process. However, in recent years, correlation study results have also shown that caspases have important roles in cell differentiation as well [31,32]. Studies of the osteoblast differentiation process have showed that excessive activation and expression of caspases are involved in the induction of differentiation of MC3T3-E1 bone cells by Bone Morphogenetic Protein (BMP)-4, while apoptotic or necrotic cells were not observed [25,31,32]. Specific inhibitors of caspases 2, 3, and 8, as well as a broad-spectrum caspase inhibitor, have also been shown to inhibit the biological activity of alkaline phosphatase. In addition, parathyroid hormone levels were found to correlate with cAMP expression levels in MC3T3-E1 cells treated with BMP4, thereby revealing a possible mechanism for the observed inhibition of osteoblast function. Thus, caspase-3 appears to play a very crucial role in maintaining gene expression in osteoblasts.
In previous studies, L-NMMA was found to significantly reduce the expression level of caspase-3 in articular cartilage, to up-regulate the ratio of Bcl-2/Bax, and to inhibit the apoptosis of articular chondrocytes in a dose-dependent manner. In the present study, the effect of L-NMMA on the expression levels of caspase-3 and caspase-9 during apoptosis induced by serum starvation was investigated. In our preliminary experiments, 50 μM L-NMMA was found to significantly reduce the expression levels of caspase-3 and caspase-9 in articular cartilage. Subsequently, L-NMMA treatment was found to decrease the elevated expression levels of caspase-3 and caspase-9 due to apoptosis induced by serum starvation. Thus, it appears that caspase-3 and caspase-9 are apoptotic effectors of NO-mediated apoptosis in NP cells. This is consistent with the hypothesis that both Bax and caspase-3 have important roles in mediating apoptosis in NP cells in human cervical discs [33]. Furthermore, the observations that caspase-9 is an initiating factor for activation of the mitochondrial apoptotic pathway, is an essential factor in the death receptor-dependent pathway, and is a key protease in the mitochondrial apoptosis pathway, supports this hypothesis [34].
NO generated by endogenous NOS enzymes or NO donors have been found to promote or prevent apoptosis induced by diverse pro-apoptotic stimuli in cell culture models [35]. In general, low doses of NO production mediate a protective effect in apoptosis, while high doses of NO production promote apoptosis. Both mitochondrial-dependent and mitochondrial-independent apoptotic signaling pathways are involved in mediating this dichotomous cellular response to NO and the molecular mechanisms responsible for these effects are complex [36]. Elevated levels of NO can induce apoptosis by mediating oxidative stress, by interfering with energy metabolism, by directly damaging DNA, by activating poly-ADP polymerase, or by inducing Ca2+ disorder [35]. In the present study, inhibition of NO production by L-NMMA in NP cells led to a marked inhibition of the decrease in the Bcl-2/Bax ratio induced by serum starvation. Increases in capase-3 and caspase-9 expression levels were also inhibited. Taken together, these results clearly indicate that NO regulates the expression of apoptotic proteins involved in mediating the apoptosis process in NP cells. However, the exact mechanism by which NO regulates the Bcl/Bax ratio and the expression levels of certain caspases remains to be elucidated.
In conclusion, the isolation and treatment of primary NP cells with L-NMMA was performed to inhibit NO production and to investigate the effects of NO, and the associated mechanism(s), on serum-induced apoptosis in NP cells cultured in vitro. When NO production was inhibited by L-NMMA, serum starvationinduced apoptosis was delayed in NP cells, concomitant with blocking of the decrease in the Bcl-2/Bax ratio and increased expression levels of caspase-3 and caspase-9. Thus, inhibition of apoptosis by NP cells may represent a novel treatment approach for delaying intervertebral disc degeneration.
Acknowledgement
This research was funded by Qingyuan People’s Hospital.
Disclosure of Conflict of Interest
The authors have no conflicts of interest.
References
- Salagnac JM, Delaire J, Mercier J. Vertical development of the face and cervical spine. Diagnostic and therapeutic significance in orthodontics and maxillofacial surgery. Rev Stomatol Chir Maxillofac 1999; 100: 13-26.
- Shibuki K, Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature 1991; 349: 326-328.
- Palmer RM. The L-arginine: nitric oxide pathway. Curr Opin Nephrol Hypertens 1993; 2: 122-128.
- Benzing WC, Mufson EJ. Increased number of NADPH-d-positive neurons within the substantia innominata in Alzheimer's disease. Brain Res 1995; 670: 351-355.
- Asahara H, Yokoi I, Tamada T, Kabuto H, Ogawab N, Mori A. Increased cerebrospinal fluid nitrite and nitrate levels in patients with lumbar spondylosis. Res Commun Mol Pathol Pharmacol 1996; 91: 77-83.
- Kimura S, Watanabe K, Yajiri Y, Motegi T, Masuya Y, Shibuki K. Cerebrospinal fluid nitric oxide metabolites in painful diseases. Neurorep 1999; 10: 275-279.
- Smith LJ, Chiaro JA, Nerurkar NL, Cortes DH, Horava SD, Hebela NM. Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following long-term agarose culture. Eur Cell Mater 2011; 22: 291-301.
- Adams MA, McNally DS, Dolan P. 'Stress' distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br 1996; 78: 965-972.
- Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004; 29: 2691-2699.
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 1996; 98: 996-1003.
- Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatol 2008; 47: 809-814.
- Ding F, Shao ZW, Xiong LM. Cell death in intervertebral disc degeneration. Apoptosis 2013; 18: 777-785.
- Burke JG, RW GW, Conhyea D, McCormack D, Dowling FE, Walsh MG. Human nucleus pulposis can respond to a pro–inflammatory stimulus. Spine (Phila Pa 1976) 2003; 28: 2685-2693.
- Wang IC, Ueng SW, Lin SS, Niu CC, Yuan LJ, Su CI. Effect of hyperbaric oxygenation on intervertebral disc degeneration: an in vitro study with human lumbar nucleus pulposus. Spine (Phila Pa 1976) 2011; 36: 1925-1931.
- Wu GJ, Chen TG, Chang HC, Chiu WT, Chang CC, Chen RM. Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. J Cell Biochem 2007; 101: 1520-1531.
- Gruber HE, Hanley EN Jr. Ultrastructure of the human intervertebral disc during aging and degeneration: comparison of surgical and control specimens. Spine (Phila Pa 1976) 2002; 27: 798-805.
- Park JB, Kim KW, Han CW, Chang H. Expression of Fas receptor on disc cells in herniated lumbar disc tissue. Spine (Phila Pa 1976) 2001; 26: 142-146.
- Gruber HE, Hanley EN Jr. Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine (Phila Pa 1976) 1998; 23: 751-757.
- Kim KW, Ha KY, Lee JS, Rhyu KW, An HS, Woo YK. The apoptotic effects of oxidative stress and anti-apoptotic effects of caspase inhibitors on rat notochordal cells. Spine (Phila Pa 1976) 2007; 32: 2443-2448.
- Park JB, Lee JK, Park SJ, Kim KW, Riew KD. Mitochondrial involvement in fas-mediated apoptosis of human lumbar disc cells. J Bone Joint Surg Am 2005; 87: 1338-1342.
- Gruber HE, Hanley EN Jr. Human disc cells in monolayer vs. 3D culture: cell shape, division and matrix formation. BMC Musculoskelet Disord 2000; 1: 1.
- Lee JY, Hall R, Pelinkovic D, Cassinelli E, Usas A, Gilbertson L. New use of a three-dimensional pellet culture system for human intervertebral disc cells: initial characterization and potential use for tissue engineering. Spine (Phila Pa 1976) 2001; 26: 2316-2322.
- Gruber HE, Ma D, Hanley EN Jr, Ingram J, Yamaguchi DT. Morphologic and molecular evidence for gap junctions and connexin 43 and 45 expression in annulus fibrosus cells from the human intervertebral disc. J Orthop Res 2001; 19: 985-989.
- Gruber HE, Norton HJ, Ingram JA, Hanley EN Jr. The SOX9 transcription factor in the human disc: decreased immunolocalization with age and disc degeneration. Spine (Phila Pa 1976) 2005; 30: 625-630.
- He Y, Qiu Y, Zhu F. Quantitative analysis of types I and II collagen in the disc annulus in adolescent idiopathic scoliosis. Stud Health Technol Inform 2006; 123: 123-128.
- Gan JC, Ducheyne P, Vresilovic EJ, Shapiro IM. Intervertebral disc tissue engineering II: cultures of nucleus pulposus cells. Clin Orthop Relat Res 2003; 411: 315-324.
- Maeda S, Kokubun S. Changes with age in proteoglycan synthesis in cells cultured in vitro from the inner and outer rabbit annulus fibrosus. Responses to interleukin-1 and interleukin-1 receptor antagonist protein. Spine (Phila Pa 1976) 2000; 25: 166-169.
- Wang W, Passaniti A. Extracellular matrix inhibits apoptosis and enhances endothelial cell differentiation by a Nf kappa B-dependent mechanism. J Cell Biochem 1999; 73: 321-331.
- Selander KS, Monkkonen J, Karhukorpi EK, Harkonen P, Hannuniemi R, Vaananen HK. Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages. Mol Pharmacol 1996; 50: 1127-1138.
- Lu L, Osmond DG. Apoptosis and its modulation during B lymphopoiesis in mouse bone marrow. Immunol Rev 2000; 175: 158-174.
- Hay E, Lemonnier J, Fromigue O, Marie PJ. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase C-dependent signaling pathway. J Biol Chem 2001; 276: 29028-29036.
- Hay E, Lemonnier J, Fromigue O, Guenou H, Marie PJ. Bone morphogenetic protein receptor IB signaling mediates apoptosis independently of differentiation in osteoblastic cells. J Biol Chem 2004; 279: 1650-1658.
- Mogi M, Togari A. Activation of caspases is required for osteoblastic differentiation. J Biol Chem 2003; 278: 47477-47482.
- Cain K, Bratton SB, Langlais C, Walker G, Brown DG, Sun XM. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J Biol Chem 2000; 275: 6067-6070.
- Boyd CS, Cadenas E. Nitric oxide and cell signaling pathways in mitochondrial–dependent apoptosis. Biol Chem 2002; 383: 411-423.
- Ding F, Shao ZW, Yang SH, Wu Q, Gao F, Xiong LM. Role of mitochondrial pathway in compression-induced apoptosis of nucleus pulposus cells. Apoptosis 2012; 17: 579-590.