ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2011) Volume 22, Issue 3
Inhibition of interleukin 17 production by curcumin in mice with collagen-induced arthritis
Laboratory of Immunology and Microbiology, Faculty of Pharmacy, Chiba Institute of Science, 3 Shiomi-cho, Choshi, Chiba 288-0025, Japan
- *Corresponding Author:
- Yoshihiro Okamoto
Laboratory of Immunology and Microbiology
Faculty of Pharmacy, Chiba Institute of Science
3 Shiomi-cho, Choshi, Chiba 288-0025, Japan
Accepted May 08 2011
Autoimmune inflammatory diseases, such as rheumatoid arthritis (RA), have been thought to be mediated by the cytokine interleukin 17 (IL-17). Curcumin (1,7-Bis (4-hydroxy-3-methoxyphenyl)-1,6 heptadiene- 3, 5-di-one) is an active ingredient derived from rhizomes of Curcuma longa Linn., displaying remarkable anti-inflammatory. Curcumin has been re-ported to have the suppressive effect on the development of an experimental animal model of RA, collagen-induced arthritis (CIA). However, there is no evidence for the effect of cur-cumin on IL-17 production of CIA mice. In the present study, we demonstrated that curcu-min inhibited the production of IL-17 in vitro. The treatments of curcumin exhibited an in-hibitory activity against CIA. We further demonstrated that curcumin suppressed IL-17 production of CIA mice using enzyme-linked immunospot assay and intracellular cytokine staining. We reported that curcumin had the inhibitory activity on IL-17 production in CIA mice. The suppression of CIA might result from the inhibition of IL-17 production.
Keywords
Curcumin, interleukin-17 (IL-17), collagen-induced arthritis (CIA)
Introduction
Previous studies have revealed that treatment with a neu-tralizing interleukin (IL) -17 antibody after the onset of collagen induced arthritis (CIA), which is an experimental animal model of rheumatoid arthritis (RA), significantly reduces the severity of CIA [1]. IL-17-deficient mice are resistant to the development of CIA [2]. Moreover, high level of IL-17 expression is detected in the target tissues during the progression of various human autoimmune diseases [3]. These data suggest that IL-17 plays a key role in the induction and propagation of autoimmunity [4].
Curcumin (1,7-Bis (4-hydroxy-3-methoxyphenyl)-1,6 he-ptadiene-3,5-di-one) is an active ingredient derived from rhizomes of Curcuma longa Linn., displaying remarkable antiinflammatory and antiarthritic activities. These bene-ficial effects have been attributed to the capacity of cur-cumin to prevent activation of nuclear factor-kappa B and the subsequent overexpression of proinflammatory media-tors; cytokines, adhesion molecules, cyclooxygenase-2, phospholipase A2, myeloperoxidase, collagenase, as well as to its ability to modulate activities of T lymphocytes and macrophages [5-8].
Furthermore, curcumin might have the inhibitory effect on the development of CIA [9,10]. However, it is unclear whether the inhibitory effect against CIA is related with IL-17 production. As IL-17 plays a very important role in process of the autoimmune arthritis, we wondered whether curcumin has any effects on IL-17 production in CIA mice.
In the present study, we demonstrated that curcumin di-rectly and significantly inhibited production of IL-17 in vitro. Moreover, we examined that the effect of curcumin on an experimental animal model of RA [11], which was IL-17 mediated autoimmune disease [3].
Materials and Methods
Animals
Six-week-old female DBA/1J mice were purchased from Nihon SLC, Shizuoka, Japan. All experiments were con-ducted in accordance with the institutional ethical guide-lines for the care and use of laboratory animals of Chiba Institute of Science.
Collagen-induced arthritis
Native bovine type II collagen (BCII, Chondrex, Red-mond, WA, USA) was emulsified in complete Freund’s adjuvant (CFA, Chondrex, USA). The mice had 100 μg BCII in CFA injected subcutaneously to the base of the tail. A booster injection containing 100 μg emulsified BCII in incomplete Freund’s adjuvant (IFA, Chondrex) was given intraperitoneally on day 21 after the first im-munization [11]. Arthritis scores were assigned according to Wood’s assessment [12]. All paws except the injected paw were graded from 0 to 4 points. The score was de-fined as the sum of the scores of all paws of each mouse.
Curcumin treatments of CIA mice
Curcumin (Sigma-Aldrich, St. Louis, MO, USA) was dis-solved in 0.4 % methylcellulose (Shin-Etsu Chemical In-dustry, Co., Ltd., Tokyo, Japan), and orally administered to the mice at 100 mg/kg daily from day 21 until the end of the experiment (day 33). Mice in the control group re-ceive equal volume of 0.4 % methylcellulose solution.
Preparation of splenocytes
The mice were sacrificed by cervical spine dislocation. After they had been sacrificed, their spleens were asepti-cally removed and crushed into a single cell suspension, and their red blood cells were lysed with Tris-buffered ammonium chloride. The single-cell suspensions were prepared in RPMI 1640 medium containing 10 % heat-inactivated fetal calf serum (FCS, Invitrogen, Life Tech-nologies Co., Carlsbab, CA, USA).
Detection of cytokine production by ELISA
Splenocytes (2x106 cells/ml) were stimulated with phor-bol myristate acetate (PMA, 0.05 μg/ml) and ionomycin (1.μg/ml) at 37 °C for 48 hours, and the concentrations of IL-17A in the supernatants were determined by ELISA (R&D Systems Inc., Minneapolis, MN, USA), according to the manufacturer’s instructions.
RT-PCR analysis of IL-17 mRNA expression
Single cell suspensions (1x106 cells/ml) of each group were stimulated with PMA (0.05 μg/ml) and ionomycin (1. μg/ml) for 5 hours. The total RNA of the cells was iso-lated by the acid guanidinium thiocyanate-phenol-chloroform extraction method using RNAiso plus (Takara Inc, Kyoto, Japan). RNA was then reverse transcribed to cDNA using reverse transcriptase (ReverTra Ace, Toyobo, Osaka, Japan) with Oligo dT primer. The real-time PCR mixture consisted of 2.5. μl of SYBR Green Buffer, 2mM MgCl2, 1mM dNTP, 0.01U/ml AmpErase uracil N-glycosylase, 0.05ŨAmpliTaq Gold (Applied Biosys-tems, part of Life Technologies Co., Carlsbad, CA, USA), forward and reverse primers (200 nM for IL-17A and glyceraldehyde-3-phosphate dehydrogenase, GAPDH), and 2 μl cDNA samples in a total volume of 25 μl. The primer sequences are shown in Table 1. The PCR reac-tions were performed in a MicroAmp optical 96 well re-action plate for 40 cycles (95°C for 15 seconds, 60 °C for 1 minute) in the ABI Prism 7500 Sequence Detector (Ap-plied Biosystems). The fluorescent signals (δCt) detected during the threshold cycle were recorded by the software installed on the machine. To standardize the target gene level with respect to the variability in RNA and cDNA quality, GAPDH was amplified under the same conditions as an internal control.
Intracellular cytokine staining
Splenocytes (2x106 cells/ml) were stimulated with PMA (0.05 μg/ml) and ionomycin (1 μg/ml) for 4 hours and added brefeldin A (BFA, 5μg/ml) for the last 2 hours of each culture. Cells were first stained extracellular, fixed and permeabilized, and then stained intracellulary accord-ing to previous report [13].The following antibodies were used :spectral red (SPRD)-labeled anti-CD4, fluorescein isothiocyanate (FITC)-labeled anti-IL-17A (eBioscience, San Diego, CA, USA). Isotype antibody with matched fluorochromes was used as controls. Samples were ac-quired on Epics ALTRA (Beckman Coulter, Fullerton, CA, USA) and data were analyzed with EXPO32 Analysis (Beckman Coulter).
Cytokine ELISPOT assay
To evaluate the number of IL-17 producing cells, an en-zyme-linked immunospot (ELISPOT) assay was per-formed [14]. Splenocytes (2x106 cells/ml) were stimulated with PMA (0.05 μg/ml) and ionomycin (1.μg/ml) at 37 °C for 5 hours. The stimulated cells were recovered and added to a plate (MultiScreen Filter plates, Millipore Cor-poration, Bedford, MA, USA) that had been coated with 50 μl of 4 μg/ml monoclonal rat anti-mouse IL-17A (R&D Systems Inc., MN, USA) in carbonate buffer and incubated overnight at 4 °C . The plate was then incu-bated at 37 °C for 12 hours. Thereafter, the plate was washed, and biotinylated goat anti-mouse IL-17A anti-body (50 μl, 300 ng/ml, R&D Systems Inc.) was added, before the plate was incubated for 2 hours at room tem-perature. The plates were then washed, and 50.μl of HRP-conjugated anti-biotin goat polyclonal antibody (1/5000, Vector Laboratories, Inc.) were added, and then the plates were incubated for 1 hour at room temperature.
The plate was then washed and 50 μl of 3-amino-9-ethylcarbazole (AEC) substrate (Vector Laboratories, INC., CA, USA) were added. The developed spots were then enumerated under low magnification (x40) with a microscope.
Results
The effect of curcumin on the IL-17 production of splenocytes
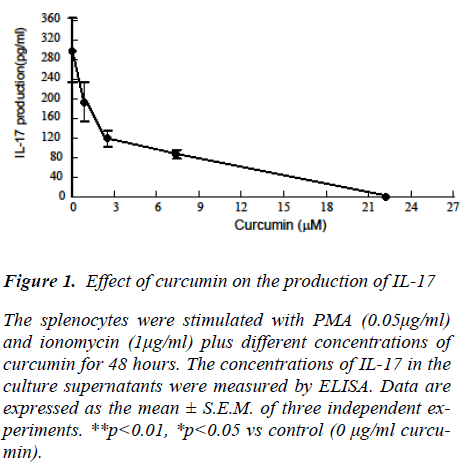
To examine the direct effect of curcumin on the IL-17 production of spleen cells of normal mice, splenocytes were stimulated with Concanavalin A (ConA) in the pres-ence of various concentrations of curcumin (Fig. 1). Dose-dependent declines in IL-17 production were ob-served. DMSO solvent (0.01%) of curcumin did not affect cytokine production (data not shown). The inhibitory ac-tivity of curcumin could not be attributed to its cytotoxic-ity, because curcumin concentrations that suppressed ConA-induces IL-17 production did not affect cell viabil-ity (data not shown).
Figure 1: Effect of curcumin on the production of IL-17 The splenocytes were stimulated with PMA (0.05μg/ml) and ionomycin (1μg/ml) plus different concentrations of curcumin for 48 hours. The concentrations of IL-17 in the culture supernatants were measured by ELISA. Data are expressed as the mean ± S.E.M. of three independent ex-periments. **p<0.01, *p<0.05 vs control (0 μg/ml curcu-min).
The effect of curcumin treatment on CIA mice
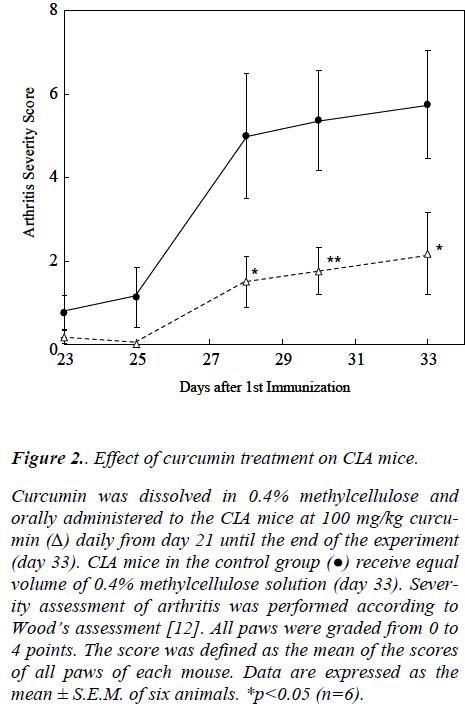
As shown in Fig. 2, the curcumin-treated mice exhibited statistically significant lower arthritis scores than the con-trol mice. Thirteen days exposure to curcumin did not affect the growth of the mice, as determined by their body weight (data not shown).
Figure 2: Effect of curcumin treatment on CIA mice.
Curcumin was dissolved in 0.4% methylcellulose and orally administered to the CIA mice at 100 mg/kg curcu-min (Δ) daily from day 21 until the end of the experiment (day 33). CIA mice in the control group (●) receive equal volume of 0.4% methylcellulose solution (day 33). Sever-ity assessment of arthritis was performed according to Wood’s assessment [12]. All paws were graded from 0 to 4 points. The score was defined as the mean of the scores of all paws of each mouse. Data are expressed as the mean ± S.E.M. of six animals. *p<0.05 (n=6).
Cytokine production of spleen cells in CIA mice
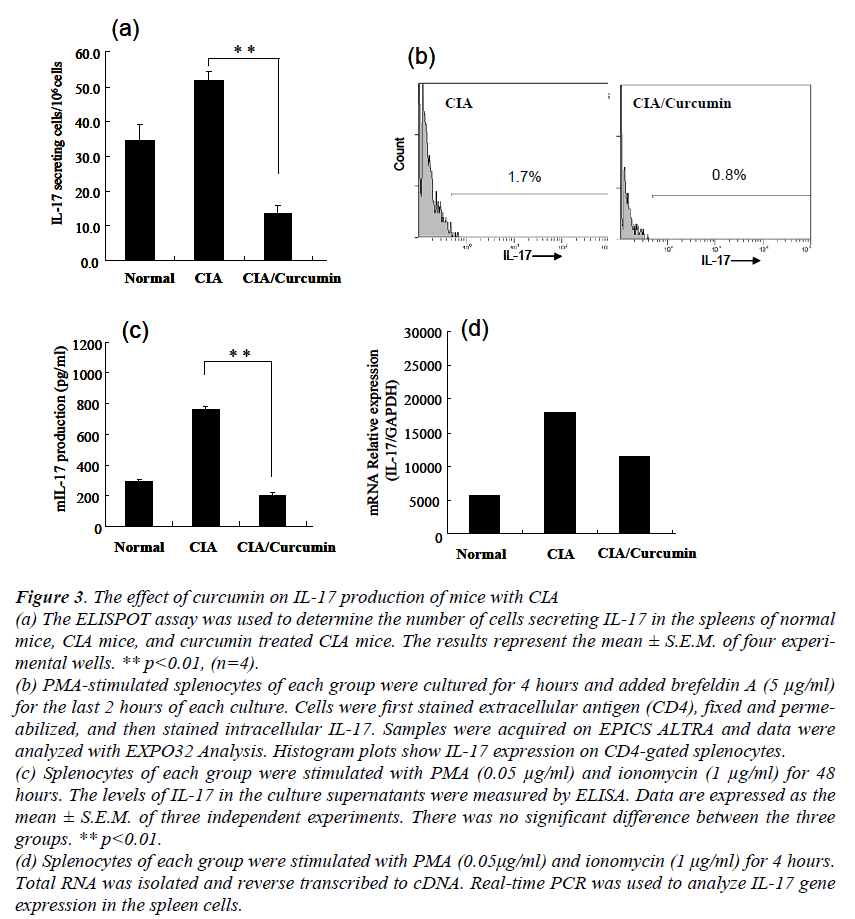
To determine how curcumin affect IL-17 production in CIA mice, intracellular cytokine staining and ELISPOT was used to detect IL-17 producing cells. ELISPOT assay showed that statistically significant decreases in the num-bers of IL-17 producing cells were observed in the cur-cumin treated CIA mice (Fig. 3a). The flow-cytometric analysis also demonstrated the decreased number of IL-17 positive cells in the splenocytes of the curcumin treated CIA mice (Fig. 3b).
Figure 3: The effect of curcumin on IL-17 production of mice with CIA (a) The ELISPOT assay was used to determine the number of cells secreting IL-17 in the spleens of normal mice, CIA mice, and curcumin treated CIA mice. The results represent the mean ± S.E.M. of four experi-mental wells. ** p<0.01, (n=4). (b) PMA-stimulated splenocytes of each group were cultured for 4 hours and added brefeldin A (5 μg/ml) for the last 2 hours of each culture. Cells were first stained extracellular antigen (CD4), fixed and perme-abilized, and then stained intracellular IL-17. Samples were acquired on EPICS ALTRA and data were analyzed with EXPO32 Analysis. Histogram plots show IL-17 expression on CD4-gated splenocytes. (c) Splenocytes of each group were stimulated with PMA (0.05 μg/ml) and ionomycin (1 μg/ml) for 48 hours. The levels of IL-17 in the culture supernatants were measured by ELISA. Data are expressed as the mean ± S.E.M. of three independent experiments. There was no significant difference between the three groups. ** p<0.01. (d) Splenocytes of each group were stimulated with PMA (0.05μg/ml) and ionomycin (1 μg/ml) for 4 hours. Total RNA was isolated and reverse transcribed to cDNA. Real-time PCR was used to analyze IL-17 gene expression in the spleen cells.
Furthermore, to analyze their cytokine production, the splenocytes of each group were stimulated with PMA and ionomycin for 48 hours.
The levels of IL-17 in the supernatants were quantified by ELISA (Fig. 3c). A significant difference between the non treatment CIA mice and the curcumin treated CIA mice was observed in IL-17 production (p<0.01). Furthermore, the collagen specific IL-17 production in the curcumin treated CIA mice also decreased significantly (data not shown).
Discussion
Some studies in vivo and in vitro indicated that curcumin is a potent anti-inflammatory agent [15]. In this study, we reported mechanisms underlying anti-inflammatory ac-tions of curcumin in CIA.
We showed the IL-17 production of spleen cells in vitro was significantly decreased in a dose dependent manner by the addition of curcumin (Fig. 1). Curcumin might have a suppressive activity on IL-17 production. IL-17 has been reported to aggravate the symptoms of the ex-perimental arthritis mice [2,16,17]. Thus, we examined that curcumin would be able to reduce the severity of the experimental arthritis model. Treatment of mice with cur-cumin decreased the clinical symptoms of CIA (Fig. 2).
The results show that curcumin has the inhibitory effect on the development of CIA, and agree with the previous studies [9,10].
To assess IL-17 producing cells in CIA mouse, ELISPOT assay (Fig. 3a) and intracellular IL-17 staining (Fig. 3b) were performed. A significant decrease of IL-17 produc- ing cells in the curcumin-treated CIA mice was observed. This suggests that curcumin inhibits IL-17 production in CIA mice.
The detail mechanisms of RA remain unknown. After 2 decades after proposal of the Th1/Th2 paradigm[18,19], Th17, a new subset of helper T cells has entered the field of autoimmunity[16,20]. Recent researches showed de-fective Th17 differentiation do not develop CIA [2,21].
Curcumin is also known for the specific inhibitor of nu-clear factor KB (NF-KB) and subsequent anti-inflamma-tory activity [22,23]. NF-B/Rel proteins are ubiquitous transcription factors that are activated in T lymphocytes and regulate the immune response and the pathogenesis of autoimmune arthritis [24].
STAT3 and NF-KB signal pathway is required for IL-17 expression [25]. The inhibitory action of NF-KB [22,23] and STAT3 [26-28] by curcumin may be related to its re-duction of IL-17 production.
In summary, we showed that curcumin directly and sig-nificantly inhibited production of IL-17 by splenocytes. Furthermore, we demonstrated for the first time that cur-cumin had the inhibitory effect on the development of CIA by the supression of IL-17 production.
Acknowledgements
The authors would like to acknowledge the financial sup-ports rendered by the Urakami Foundation (2009).
References
- Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joos- ten LA, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum 2004;50(2):650-9.
- Nakae S, Nambu A, Sudo K, Iwakura Y. Sup- pression of immune induction of collagen- induced arthritis in IL-17-deficient mice. J Im- munol 2003;171(11):6173-7.
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis ofT cell-mediated tissue damage. Nat Med 2007;13(2):139-45.
- Lubberts E. Th17 cytokines and arthritis.Semin Immunopathol 2010;32(1):43-53.
- Sharma RA, Gescher AJ, Steward WP. Curcu- min: the story so far. Eur J Cancer2005;41(13):1955-68.
- Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, et al. Turmeric extracts contain- ing curcuminoids prevent experimental rheu- matoid arthritis. J Nat Prod 2006;69(3):351-5.
- Jagetia GC, Aggarwal BB. "Spicing up" of the immune system by curcumin. J Clin Immunol2007;27(1):19-35.
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinicalstudies. Anticancer Res 2003;23(1A):363-98.
- Moon DO, Kim MO, Choi YH, Park YM, Kim GY. Curcumin attenuates inflammatory re-sponse in IL-1beta-induced human synovial fi- broblasts and collagen-induced arthritis in mouse model. Int Immunopharmacol2010;10(5):605-10.
- Mun SH, Kim HS, Kim JW, Ko NY, Kim do K, Lee BY, et al. Oral administration of curcuminsuppresses production of matrix metallopro-teinase (MMP)-1 and MMP-3 to ameliorate col- lagen-induced arthritis: inhibition of thePKCdelta/JNK/c-Jun pathway. J Pharmacol Sci2009;111(1):13-21.
- Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterolo- gous type II collagen induces arthritis in mice. Nature 1980;283(5748):666-8.
- Wood FD, Pearson CM, Tanaka A. Capacity of mycobacterial wax D and its subfractions to in-duce adjuvant arthritis in rats. Int Arch Allergy Appl Immunol 1969;35(5):456-67.
- Pappu BP, Dong C. Measurement of inter- leukin-17. Curr Protoc Immunol 2007;Chapter 6: Unit 6 25.
- Okamoto Y, Abe T, Niwa T, Mizuhashi S, Ni- shida M. Development of a dual color enzyme- linked immunospot assay for simultaneous de-tection of murine T helper type 1- and T helper type 2-cells. Immunopharmacology 1998; 39 (2):107-116.
- Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci 2006;78(18):2081-7.
- Harrington LE, Mangan PR, Weaver CT. Ex- panding the effector CD4 T-cell repertoire: theTh17 lineage. Curr Opin Immunol 2006; 18(3): 349-56.
- Yamaguchi Y, Fujio K, Shoda H, Okamoto A, Tsuno NH, Takahashi K, et al. IL-17B and IL- 17C are associated with TNF-alpha productionand contribute to the exacerbation of inflamma- tory arthritis. J Immunol 2007;179(10):7128-36.
- Street NE, Mosmann TR. Functional diversity of T lymphocytes due to secretion of differentcytokine patterns. Faseb J 1991;5(2):171-7.
- Mosmann TR, Coffman RL. TH1 and TH2 cells:different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145-73.
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005;6(11):1123-32.
- Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cyto-kine 2008;41(2):84-91.
- Bengmark S, Mesa MD, Gil A. Plant-derived health: the effects of turmeric and curcuminoids.Nutr Hosp 2009;24(3):273-81.
- Bremner P, Heinrich M. Natural products as targeted modulators of the nuclear factor-kappaB pathway. J Pharm Pharmacol2002;54(4):453-72.
- Seetharaman R, Mora AL, Nabozny G, Boothby M, Chen J. Essential role of T cell NF-kappa Bactivation in collagen-induced arthritis. J Im- munol 1999;163(3):1577-83.
- Cho ML, Kang JW, Moon YM, Nam HJ, JhunJY, Heo SB, et al. STAT3 and NF-kappaB sig- nal pathway is required for IL-23-mediated IL- 17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol 2006;176(9):5652-61.
- Blasius R, Reuter S, Henry E, Dicato M, Died- erich M. Curcumin regulates signal transducerand activator of transcription (STAT) expres-sion in K562 cells. Biochem Pharmacol 2006;72(11):1547-54.
- Liu YC, Hsieh CW, Wu CC, Wung BS. Chalconeinhibits the activation of NF-kappaB and STAT3 in endothelial cells via endogenous elec- trophile. Life Sci 2007;80(15):1420-30.
- Xie L, Li XK, Funeshima-Fuji N, Kimura H, Matsumoto Y, Isaka Y, et al. Amelioration of experimental autoimmune encephalomyelitis bycurcumin treatment through inhibition of IL-17 production. Int Immunopharmacol 2009;9(5):575-81.