ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 5
Implication of autophagy and apoptosis in spiral ganglion cells and cochlear nucleus nuerons in diabetes-induced hearing impairment in rats
1Department of Otolaryngology, Head and Neck Surgery, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, PR China
2Department of Otolaryngology, the Second School of Clinical College, Guangdong Medical University, Dongguan, Guangdong, PR China
3Guangdong Provincial Key Laboratory of Medical Molecular Diagnostics, Guangdong Medical University, Dongguan, Guangdong, PR China
- *Corresponding Author:
- Liang Yong
Department of Otolaryngology Head and Neck Surgery, Nanfang Hospital
Southern Medical University, PR China
Accepted date: October 13, 2016
Purpose: This study was carried out to investigate incidence of autophagy and apoptosis in spiral ganglion cells (SGCs) and cochlear nucleus neurons, and their involvement in the pathogenesis of diabetes-induced hearing impairment in rats.
Methods: Rats were randomly divided into control group and diabetes groups, with variations in duration of diabetes: 4 weeks (DM4W), 8 weeks (DM8W) and 12 weeks (DM12W). Diabetes was induced by intraperitoneal injection of streptozotocin. The rats of each group were tested with auditory brainstem response (ABR). Ultra-structures of cochlear nuclei and SGCs were examined by transmission electron microscope. Western blot and immunehistochemical methods were used to for estimating the expression of autophagy-related factors (Beclin 1 and cleaved-LC3); apoptosis-related factors (Bax, Bcl-2 and cleaved Caspase-3 protein) in cochlear nuclei and SGCs.
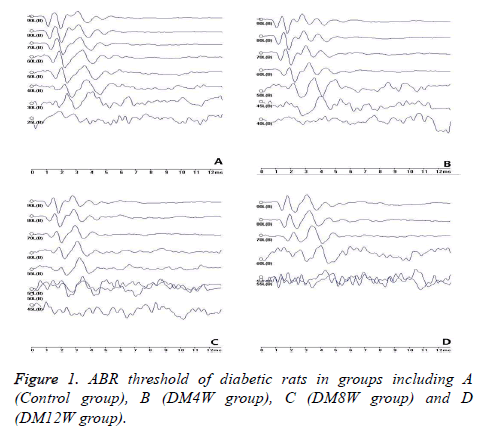
Results: ABR response thresholds of rats in DM8W and DM12W group were increased significantly. Compared to control group, the latency and wave duration of DM12W group were higher. Mitochondria swelling and vacuoles were observed in SGCs of diabetes group. More lysosomes and myelin layer plate fractures were seen in cochlear nuclei of diabetes group. The expression of Beclin 1, cleaved-LC3, Bax and cleaved Caspase-3 protein in DM8W and DM12W groups increased, while expression of Bcl-2 was decreased, both significantly.
Conclusion: The results show that autophagy and apoptosis are implicated in the development of hearing impairment in diabetic ratss.
Keywords
Diabetes, Hearing impairment, Autophagy, Apoptosis
Introduction
Diabetes can cause varying degrees of damage to auditory function, which manifests clinically as a slow-onset, bilateral, progressive and symmetry sound nerve deafness. The severity of hearing loss is related to the duration of diabetes [1,2]. Cell autophagy is a degradative lysosomal process which is highly conserved evolutionally in eukaryotic cells [3]. Autophagy and apoptosis can be mutually activated, and share multiple molecular switches and coordinate transformation [4-7]. In recent years, studies have shown that autophagy and apoptosis are correlated with occurrence and development of diabetic complications such as diabetic nephropathy [8,9] and diabetic cardiomyopathy [10-12]. However, reports relating autophagy to diabetes-induced hearing impairment. The influence of diabetes on chronic pathological changes of auditory system was investigated in this study. Apoptosis and autophagy were studied to see whether they are involved in the high blood sugar-induced toxicity to SGCs and cochlear nuclear neurons.
Material and Methods
Animals
Male Sprague-Dawley rats (n=70), weighing 300~350 g were supplied by Experimental Animal Center of Southern Medical University (Address: No.1023 Shatai Nan Road, Baiyun District, Guangzhou, Guangdong, China). Auricle responses, electric otoscopy and tympanic membranes of the animals were normal. Their random peripheral blood glucose levels were within the normal range.
Preparation of diabetic rat model and animal grouping
The diabetic model group was developed after 1 week of acclimatization of the rats. The rats were fasted for 12 h prior to induction of diabetes by intraperitoneal injection of 1% streptozotocin solution (STZ, 55 mg/kg, single dose). The control group was injected with equivalent volume of citric acid buffer solution [13]. Water and feed were provided ad libitum to the rats. Blood was taken via tail vein 72 h after injection of streptozotocin. Fasting blood sugar was measured with Roche glucose meter. Rats with blood sugar levels>16.7 mmol/L were used as diabetic model. The rats were randomly divided into control group, and 4-week diabetic, 8-week diabetic and 12-week diabetic groups. There were 20 rats in each diabetes group and 10 rats in control group. Fasting blood glucose and rat weights were measured once every 2 weeks.
Auditory brainstem response testing
The rats were anesthetized by intraperitoneal injection of 2% pentobarbital sodium solution at a dose of 0.3 ml/l00 g, kept warm with hot water bag. Smart EP auditory was used to test by evoking potentiometer. The record needle electrode was placed in the center of the rat parietal subcutaneous region, while the reference electrode was positioned in the ipsilateral mastoid. The grounding electrodes were placed under the right rear of the rat leg. Sound stimulation was a short click at a frequency of 11.1 times/s with duration of 0.1 ms by density and scatter alternating. Schedule of observation was 12 ms with 1024 superposition. The sound was 1 cm to outer ear through plug-in headsets. It began from 90 dB SPL. The minimum stimulus intensity of III wave in the ABR wave was determined starting with 10 dB and then reducing to 5 dB stimulation, to determine the threshold of ABR. The incubation period and the corresponding wave period of I, III, V wave were recorded at the stimulus intensity of 80 dB SPL [14]. Each test was repeated twice.
Observation of transmission electron microscopy (SEM)
The specimens were fixed with 2.5% glutaraldehyde. The cochlear was decalcified with 10% EDTA and isolated under dissecting microscope. The procedure of possessing specimens included 0.1 mol/L phosphate buffer rinsing, 1% osmic acid fixing, gradient ethanol dehydration, acetone soaking, the embedding of epoxy resin, oven-curing, block treatment, sectioning with ultra-thin slicing machine (Leica ultracut UTC, Germany) and double staining with uranium acetate and lead citrate [15]. Ultrastructural pathologies of cells in cochlear nucleus neurons and spiral ganglion were viewed under transmission electron microscope (HITACHI H-7500, Japan).
Western blot for detecting autophagy and apoptosis factors in cochlea specimen
The worm shaft and cochlear nucleus frozen at -80°C were ground in liquid nitrogen, and then 0.2 ml RIPA and 2 μl PMSF. The tissues were homogenized at low temperature with ultrasonic cell disruptor. Protein was quantified by BCA method after centrifugation. The extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and incubated at 4°C with anti-Cleaved - LC3 (dilution of 1:1000, Abclonal company, USA), anti-Cleaved Caspase-3 (dilution of 1:1000, CST company, USA); anti Beclin 1 (dilution of 1:1000, Abcam Company, UK) and anti Bax, Bcl-2 (dilution of 1:1000, Wuhan Sanying Company). After washing with TBST, they were incubated with second anti β-actin (1: 2, 000) at room temperature for 1 h. Protein bands were quantified by using grey value of Image J software after TBST catharsis and DAB coloration.
Immuno-histochemical detection of cochlear nucleus and cochlea in rats
Biopsy specimens of cochlea and cochlear nucleus were sectioned to thickness of 3 um. SABC method was used for immuno-histochemical staining according to the SABC operation manual. The primary antibody LC3 was diluted 1:700 (CST Company, US). Beclin-1 antibody was diluted 1:400 (British Abcam Company) Cleaved Caspase-3 antibody (CST Company, US). Bax antibody was diluted 1:200 and Bcl-2 antibody 1:50 (Wuhan Sanying Company). Goat anti rabbit IgG antibody was diluted 1:200 (Biyuntian Company, Shanghai). The expressions of LC3, Beclin 1, Bax, Bcl-2 and Cleaved Caspase-3 protein were observed in the cytoplasm.
Results
General observation
Compared with control group, the rats in diabetes group showed diabetic symptoms such as polyuria, polydipsia, polyphagia, weight loss, decreased activity and yellow hair. The success level achieved in induction of diabetes was 76.5%, while mortality was 22.2%. Blood glucose concentrations of rats in DM4W, DM8W and DM12W group were higher (24.16 ± 5.46, 27.38 ± 27.38 and 26.06 ± 4.44 mmol/L, respectively). Compared with the control group (6.87 ± 1.52 mmol/L), the differences are statistically significant (P<0.01). The body weight of rats in DM4, DM8W and DM12W groups were significantly lower (261.81 ± 29.01, 265.40 ± 30.89 and 263.46 ± 30.343 g, respectively). Again, compared to the control group (325.43 ± 15.41 g), the differences are statistically significant (P<0.01).
Evaluation of ABR threshold value and the duration of diabetes
Compared to the control group, ABR reaction thresholds of rats in DM8W and DM12W group were significantly increased (Figure 1). There were no significant differences in wave incubation period and wave duration between DM4W and DM8W groups (P>0.05). However, they were significantly higher in the DM12W group (P<0.05).
Ultra-structure under the transmission electron microscope
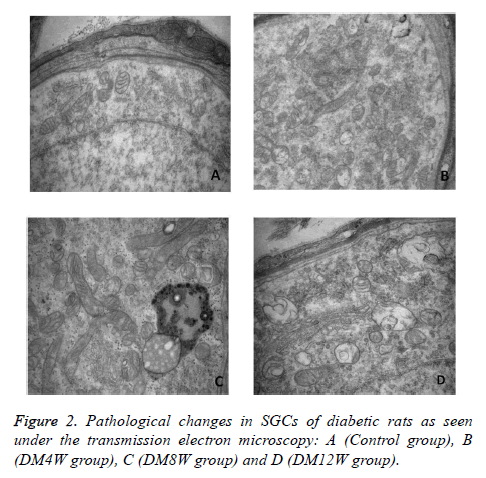
The SGCs of rats in control group appeared ovoid in shape, with clear contour of cells, normal organelles in cytoplasm and myelin plate layer arranged as concentric layers without deformation, separation or fracture (Figure 2A). The SGCs of rats in DM4W group showed a few mitochondrial swelling, vacuoles and rough endoplasmic reticulum (Figure 2B). Pathological changes in rats in DM8W and DM12W groups included fewer SGCs, reduced myelin number, autophagylysosome generation in cytoplasm, increased mitochondrial size, abnormally-shaped mitochondria, mitochondrial swelling, deformation and focal cavitation; fragmentation and disappearance of part of mitochondrial crest. These pathological changes were particularly obvious in DM12W group. The shape of myelin sheath did not show obvious abnormity (Figures 2C and 2D).
The cell morphology of cochlea in control group was normal. The shape of nerve cell body around myelin sheath was normal, with a circular and dense myelin board layer. The cochlear nucleus neurons in DM4W group had no obvious change, but more myelin distributed around the cell body had cavitation, plate layer separation and deformation. With the extension of diabetes duration, pathological changes in DM8W and DM12W groups included swelling of neurons in cochlear nuclear, mitochondrial cavitation, irregular shape of nucleus r and autophagy-lysosome. The pathological change in DM12W group was most obvious. With the progress of disease, myelin deformation, cavitation, plate layer separation and disintegration were seen.
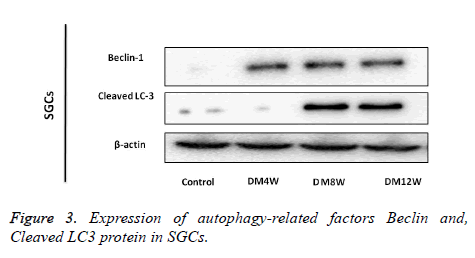
Expression of autophagy-related factors (Beclin 1and Cleaved LC3 protein) in SGCs
Beclin 1 and LC3 proteins were expressed mainly in cytoplasm of the diabetes groups. The expression level of Beclin 1 protein was higher in the groups with longer duration of diabetes. Cleaved-LC3 in DM8W and DM12W group were higher than in control group, but the level of expression of Cleaved-LC3 in DM4W group was not significantly different from that in the Control group (Figure 3).
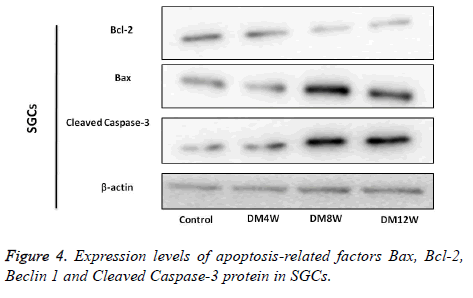
Expression of apoptosis related factors Bax, Bcl–2, Beclin 1 and Cleaved Caspase-3 protein in SGCs
There was more expression of Bax and Cleaved Caspase-3 protein in diabetes group than in control group. The positive cells of Bcl-2 in the control group were very numerous. The expression levels of Bax and Cleaved Caspase-3 protein in DM8W and DM12W group were significantly higher than in the control group. Compared with the control group, the expression of Bcl-2 protein in DM8W and DM12W group was reduced, while the expressions of Bax, BCL-2 and Cleaved Caspase-3 in DM4W group were not different from that in the control groups (Figure 4).
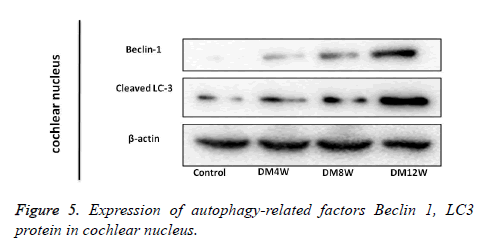
Expression of autophagy related factors Beclin 1, LC3 protein in cochlea nucleus
Expression of Beclin 1 and LC3 proteins was mainly in the cytoplasm. There were more positive cells in the diabetes group than in in control group. The expression level of Beclin 1 protein in the diabetes group differed significantly from that in the control group, but the expression of Cleaved-LC3 protein in DM4W group was not significantly different from control. However, the expressions of Cleaved-LC3 in DM8W and DM12W groups increased significantly (Figure 5).
Expression of apoptosis-related factors Bax, Bcl-2 and Cleaved Caspase-3 in the cochlear nucleus
The expressions of Bax, Bcl-2 and Cleaved Caspase-3 protein are shown in Figure 6. Compared with the control group, expression levels of Bax protein in each diabetes group was higher, while the expression of Bcl-2 protein was significantly lower. The expressions of Cleaved Caspase-3 in DM4W, DM8W and DM12W group were only slightly increased.
Discussion
SGCs as the first level neurons of auditory pathway belong to end cell with high differentiation. It is difficult to repair after injury. Auditory center is in the lower brainstem of cochlear nucleus (CN). The main function includes primary processing of voice information, faithfully spreading it to the next level nerve nuclei. Signals pass from the cochlea into the nerve center through the auditory afferent nerve. Diabetes can lead to peripheral neuropathy (DN). The pathogenesis of DN is linked to oxidative stress, inflammation, mitochondrial dysfunction, energy crisis, interaction of axons and glial cells as well as demyelinating lesions [16-18]. One of the pathological changes in DN includes peripheral neuropathy, involving myelin, axon and neurons. In the early period, it could cause focal decay of nerve axons [19], reduction of neuron dendritic branches, activation of microglia [20-22] and nerve damage. These changes may also occur in the auditory nerve system at the same time. Hearing loss in patients with diabetes may be related to the damage to cochlea. The incidence of hearing impairment in patients with diabetes ranges from 0% to 93% [23-25]. In 2014, the American Diabetes Association recommended that hearing impairment in diabetes complications be included in the diabetes diagnosis guide, and it was set as one of the areas used for diagnosis [26]. Although hearing impairment in diabetes has attracted the attention of more and more researchers, the specific mechanism involved is still unclear.
Autophagy is self-degradation of conservative cells, which are subjected to lysosomal degradation [27]. Cells play protective roles by enhancing autophagy which removes damaged organelles and metabolic products. Excessive autophagy can lead to the death of cells [28-30]. LC3 is a specific marker for detection of degree of autophagy. In some cases, the autophagy inhibits apoptosis to ensure cell survival, but it can also induce cell death by autophagy itself, or jointly with apoptosis, through multiple interactions from signalling pathways and regulatory proteins [4-7]. Bcl-2 as a protein family protein plays a dual role in controlling autophagy and apoptosis. Beclin 1 (Atg6) is the only mammalian autophagy gene, which was in homology with yeast autophagy genes. It can be combined with the Bcl-2 of anti-apoptotic proteins for regulating autophagy and apoptosis. When Beclin 1 combines with Bcl-2/Bcl-xL, the complex of Beclin 1: Bcl-2/Bcl-xL activates autophagy by inhibiting Beclin 1. When the Bcl-2 which promotes apoptosis combines with the Bcl-2/Bcl-xL, the release of Beclin 1 induces autophagy [31]. Apoptosis could also affect autophagy through caspase enzyme. Caspasemediated cleavage of Beclin 1 inactivates autophagy. When the end of the C fragment was translocated to mitochondria, it made the cell more sensitive to apoptosis [32].
Studies have confirmed that autophagy is related to chronic diabetic complications such as diabetic nephropathy [8,9], diabetic retinopathy [33], diabetic peripheral neuropathy [34,35], diabetic cardiomyopathy [10-12] and diabetes vascular lesions [36,37]. However, hearing impairment in diabetes has not been reported. This study explored pathological changes in chronic auditory system in diabetes, to see whether apoptosis and autophagy were involved in diabetes-induced damage to SGCs and toxicity of cochlear nucleus neurons. Hearing loss was associated with diabetes duration from the results of ABR. I~V wave incubation period and wave duration of ABR in the DM12W group was extended. Compared with the control group, the expression of autophagy related proteins was higher in SGCs and cochlear nucleus of diabetes group, indicating that hyperglycemia may be the factor causing increased autophagy stimulus, which may be related to the duration of disease. It was found that high blood sugar damaged the SGCs and cochlear nucleus neurons. With longer duration of diabetes, the level of apoptosis increased gradually. The results showed that autophagy and apoptosis are associated with the process of hearing impairment in rats with diabetes. Autophagy may play a role in the toxicity to SGCs and cochlear nucleus neurons through the Bcl-Beclin 1 pathway.
Conclusion
In this research, it was found that diabetes caused changes in autophagy and apoptosis in SGCs and cochlear nucleus neurons. Increase in duration of hyperglycemia led to higher autophagy and apoptosis of SGCs and cochlear nucleus neurons. The results show that autophagy and apoptosis are implicated in the development of hearing impairment in diabetic rats.
References
- Horikawa C, Kodama S, Tanaka S, Fujihara K, Hirasawa R. Diabetes and risk of hearing impairment in adults: a meta-analysis. J Clin Endocrinol Metab 2013; 98: 51-58.
- Mitchell P, Gopinath B, McMahon CM, Rochtchina E, Wang JJ. Relationship of Type 2 diabetes to the prevalence, incidence and progression of age-related hearing loss. Diabet Med 2009; 26: 483-488.
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000; 290: 1717-1721.
- Su M, Mei Y, Sinha S. Role of the Crosstalk between Autophagy and Apoptosis in Cancer. J Oncol 2013; 2013: 102735.
- Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 2014; 15: 81-94.
- Saita S, Shirane M, Nakayama KI. Selective escape of proteins from the mitochondria during mitophagy. Nat Commun 2013; 4: 1410.
- Ryter SW, Mizumura K, Choi AM. The impact of autophagy on cell death modalities. Int J Cell Biol 2014; 2014: 502676.
- Peng KY, Horng LY, Sung HC. Hepatocyte growth factor has a role in the amelioration of diabetic vascular complications via autophagic clearance of advanced glycation end products: Dispo85E, an HGF inducer, as a potential botanical drug. Metabolism 2011; 60: 888-892.
- Kitada M, Takeda A, Nagai T, Ito H, Kanasaki K. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp Diabetes Res 2011; 2011: 908185.
- Xie Z, Lau K, Eby B, Lozano P, He C. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 2011; 60: 1770-1778.
- Mellor KM, Bell JR, Young MJ, Ritchie RH, Delbridge LM. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol 2011; 50: 1035-1043.
- Younce CW, Wang K, Kolattukudy PE. Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc Res 2010; 87: 665-674.
- Wu HP, Guo YL, Cheng TJ, Hsu CJ. Chronological changes in compromised olivocochlear activity and the effect of insulin in diabetic Wistar rats. Hear Res 2010; 270: 173-178.
- Wu HP, Hsu CJ, Cheng TJ, Guo YL. N-acetylcysteine attenuates noise-induced permanent hearing loss in diabetic rats. Hear Res 2010; 267: 71-77.
- Wang Y, Wei W. Microglia-like or microglia: results of the weak silver carbonate staining method of del Rio-Hortega. Biotech Histochem 2012; 87: 346-349.
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788-824.
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010; 9: 807-819.
- Zenker J, Ziegler D, Chrast R. Novel pathogenic pathways in diabetic neuropathy. Trends Neurosci 2013; 36: 439-449.
- Bayazit YA, Goksu N. Tinnitus and neurovascular compression. ORL J Otorhinolaryngol Relat Spec 2008; 70: 209.
- Sonneville R, den Hertog HM, Güiza F, Gunst J, Derese I. Impact of hyperglycemia on neuropathological alterations during critical illness. J Clin Endocrinol Metab 2012; 97: 2113-2123.
- Malone JI, Hanna S, Saporta S, Mervis RF, Park CR. Hyperglycemia not hypoglycemia alters neuronal dendrites and impairs spatial memory. Pediatr Diabetes 2008; 9: 531-539.
- Hinder LM, Vincent AM, Burant CF, Pennathur S, Feldman EL. Bioenergetics in diabetic neuropathy: what we need to know. J Peripher Nerv Syst 2012; 17 Suppl 2: 10-14.
- Weng J, Bi Y. Diabetes in China: The challenge now. J Diabetes Investig 2010; 1: 170-171.
- Xu Y, Wang L, He J, Bi Y, Li M. Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948-959.
- Yang W, Lu J, Weng J, Jia W, Ji L. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090-1101.
- American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care 2014; 37 Suppl 1: S14-80.
- Anding AL, Baehrecke EH. Autophagy in Cell Life and Cell Death. Curr Top Dev Biol 2015; 114: 67-91.
- Mqoco T, Joubert A. 2-Methoxyestradiol-bis-sulphamate induces apoptosis and autophagy in an oesophageal carcinoma (SNO) cell line. Biomed Res 2012; 23: 469-474.
- Yamamoto S, Kazama JJ, Fukagawa M. Autophagy: a two-edged sword in diabetes mellitus. Biochem J 2013; 456: e1-3.
- Chen W, Sun Y, Liu K, Sun X. Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res 2014; 9: 1210-1216.
- He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes 2013; 62: 1270-1281.
- Kapuy O, Vinod PK, Mandl J, Bánhegyi G. A cellular stress-directed bistable switch controls the crosstalk between autophagy and apoptosis. Mol Biosyst 2013; 9: 296-306.
- Miranda S, González-Rodríguez A, García-Ramírez M Beneficial effects of fenofibrate in retinal pigment epithelium by the modulation of stress and survival signaling under diabetic conditions. J Cell Physiol 2012; 227: 2352-2362.
- Towns R, Guo C, Shangguan Y, Hong S, Wiley JW. Type 2 diabetes with neuropathy: autoantibody stimulation of autophagy via Fas. Neuroreport 2008; 19: 265-269.
- Towns R, Kabeya Y, Yoshimori T. Sera from patients with type 2 diabetes and neuropathy induce autophagy and colocalization with mitochondria in SY5Y cells. Autophagy 2005; 1: 163 -170.
- Hu P, Lai D, Lu P, Gao J, He H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med 2012; 29: 613-618.
- Zhang T, Liu X, Li Q, Wang J, Jia W. Exacerbation of ischemia-induced amyloid-beta generation by diabetes is associated with autophagy activation in mice brain. Neurosci Lett 2010; 479: 215-220.