ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 1
Hesperetin ameliorates cardiac inflammation and cardiac fibrosis in streptozotocin-induced diabetic rats by inhibiting NF-kB signaling pathway
1Department of Endocrinology, Zhongnan Hospital of Wuhan University, Wuhan 430071, Hubei Province, PR China
2Department of Endocrinology, Enshi Center Hospital, Enshi 445000, Hubei Province, PR China
- *Corresponding Author:
- Yancheng Xu
Department of Endocrinology
Zhongnan Hospital of Wuhan University
No. 169, Donghu Road, Wuhan 430071, Hubei Province
PR China
Accepted date: May 24, 2016
Objective: Activation of several signal transduction pathways, including cardiac inflammation and fibrosis, are important parts of structural and functional changes in diabetic cardiomyopathy (DCM), which is a leading cause of cardiovascular complications. NF-κB signaling pathway plays critical roles in promoting cardiac inflammation and cardiac fibrosis. It has been reported that hesperetin could inhibit cardiac remodelling induced by pressure overload in mice. However, it is still not clear whether and how hesperetin plays a role in cardiac inflammation and fibrosis in diabetic rats.
Methods: Diabetes mellitus (DM) was induced by streptozotocin (70 mg/kg body weight). Rats were received hesperetin (30 mg/kg/day) for 12 weeks. Effect of hesperetin on glycemic parameters was detected with blood glucose, glycated hemoglobin (HbA1c). After induction of diabetes, all rats were underwent echocardiography to evaluate cardiac function. Expressions of inflammation markers such as TNF-α, IL-1β, ICAM-1 and VCAM-1 were investigated by real-time PCR, gene expression of fibrosis markers such as collagen I and III, and histological analysis were applied to determine the level of cardiac fibrosis. NF-κB signaling pathway activation was also detected by western blot.
Results: Hesperetin reduced expression levels of TNF-α, IL-1β, ICAM-1, VCAM-1, collagen I and III. The level of collagen deposition in diabetic myocardium was attenuated with the treatment of hesperetin. Additionally, administration of hesperetin inhibited activation of NF-κB signaling pathway.
Conclusion: These results demonstrate that hesperetin inhibited cardiac inflammation and cardiac fibrosis post-diabetes through blocking NF-κB signaling pathway, which could be a key mechanism of hesperetin in attenuating cardiac remodeling in diabetic rats.
Keywords
Hesperetin, Cardiac inflammation, Cardiac fibrosis, NF-κb, Diabetes mellitus
Introduction
As living standard improved, diabetes mellitus (DM) has become a worldwide pandemic of the 21st century, which is one of chronic non-communicable diseases that seriously threaten to human health. Secondary to diabetes, cardiovascular complications is a major cause of diabetic patients' death and disability. Diabetic cardiomyopathy (DCM) is one of the major cardiac complications of DM patients, which is closely related to the high incidence of heart failure and death. So far, the pathogenesis of DCM exactly is unclear. Studies suggest that DCM is mostly affected by high blood glucose, energy metabolism disorder, oxidative damage, abnormal activation of neuroendocrine system, chronic inflammation, myocardial fibrosis and other factors [1-4]. In recent years, the importance of cardiac inflammation and cardiac remodelling, which have been confirmed that involved in the occurrence and development of DCM by a variety of clinical trials and basic researches [5-7]. The levels of a variety of inflammatory cytokines are significantly increased in myocardial tissue of diabetes animal model, and were positively correlated with the degree of cardiac insufficiency [8,9]. Additionally, observed in animal models of DCM, early application of anti-inflammatory therapy can inhibit the expression of inflammatory cytokines in blood circulation and heart, reduce the inflammatory response of cardiomyocytes and improve cardiac insufficiency [10,11]. Therefore, prevention of cardiac inflammation and cardiac fibrosis provides a new train of thought for treatment of DCM.
Hesperetin is the main therapeutic ingredient of the genus citrus fruit, its therapeutic effects mainly consists of anti-inflammatory, anti-oxidant, anti-apoptosis, anti-allergy, antidermatitis and anti-cancer etc. [12,13]. In the cardiovascular system, hesperetin has a certain role in dilating blood vessels, reducing blood lipid, lowering permeability of capillary and brittleness of blood vessels, inhibiting cardiac remodelling induced by pressure overload in mice confirmed in previous studies [14-16].
In view of the important roles of cardiac inflammation and fibrosis in the development of DCM and anti-inflammatory/ anti-remodeling effects of hesperetin, we speculated that hesperetin plays a protective role in DCM. This study will establish DM rats models by streptozotocin (STZ) injection, observe the effects of hesperidin on glycometabolism, cardiac function, cardiac inflammation and fibrosis, and then clarify the possible mechanism of anti-inflammatory and anti-fibrosis of hesperidin in DM rats, which tries to provide a new idea and theoretical basis for preventing diabetic cardiomyopathy.
Materials and Methods
Materials
Hesperetin (#W431300) and streptozotocin (STZ, #S0130) were purchased from Sigma-Aldrich (St. Louis, MO, USA). GAPDH (sc-365062) was obtained from Santa Cruz Biotechnology (Dallas, TX, USA). TRIZol (#15596018) was purchased from Life Technologies (Invitrogen, NY, USA). The bicinchoninic acid protein assay kit was bought from Pierce Biotechnology, Inc. (Rockford, IL, USA). Primary antibodies against NF-κB p65 (#8242) and phospho-NF-κB p65 (#3039) were purchased from Cell Signalling Technology (Danvers, MA, USA).
Streptozotocin-induced diabetic rats
The present study was conducted in accordance with the Guide for the Local Care and Use of Laboratory Animals and all procedures were performed in a blinded manner. Adult male sprague dawley rats (200-250 g) purchased from the Experimental Animal Center of Hubei Province (Wuhan, China), were randomized to undergo intraperitoneal injection of STZ (70 mg/kg body weight) in citrate buffer or control injected with the equal volume of citrate buffer [17,18]. A blood glucose concentration greater than 16.7 mmol/L in rats after 3 days and 7 days STZ injection were indicative of diabetic and included in the study for 12 weeks. As described by Deng et al. freshly 0.5% carboxymethylcellulose solution was prepared at a constant volume of 1 ml/100 g body weight by oral gavage once a day. The control group was given the same volume of liquid comprising of only the vehicle solution [16]. The rats were received with 30 mg/kg/day of hesperetin or vehicle for 12 weeks. Animals in the study were divided into four groups: vehicle-control (n=10), hesperetin-control (n=10), vehicle-DM (n=12) and hesperetin-DM (n=12).
Estimation of biochemical parameters
Effect of hesperetin on glycemic parameters were detected. Blood glucose levels in every group were measured, and HbA1c was estimated at the end of 12 weeks.
Assessments of cardiac function
After 12 weeks, cardiac function in rats was evaluated with left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) by an echocardiograph.
Histological analysis
In brief, the hearts were fixed in 10% formalin after excised from anesthetized rats. Then, the hearts were dehydrated, embedded in paraffin, sectioned transversely or longitudinally at 5 μm, and performed with standard picrosirius red (PSR) procedures for collagen deposition analysis as described in these references [19,20].
Real-time polymerase chain reaction (RT-PCR)
To evaluate the levels of TNF-α, IL-1β, ICAM-1, VCAM-1, collagen I and III, real-time polymerase chain reaction (RTPCR) analysis was used to detect the mRNA levels of the above genes. Briefly, total mRNA was extracted using TRIZol, chloroform and isopropanol etc. Synthesized cDNA was performed by using oligo (dT) primers with the Transcriptor First Strand cDNA Synthesis Kit. Selected gene differences were measured using SYBR green and normalized against gene expression of GAPDH. The sequences of primers in the study were showed as follows: TNF-α: 5'- TCCCAGAAAAGCAAGCAACC-3' and 5'- TAGACAGAAGAGCGTGGTGG-3'; IL-1β: 5'- CTCCATGAGCTTTGTACAAGG-3' and 5'- TGCTGATGTACCAGTTGGGG-3'; ICAM-1: 5’- TCGGTGCTCAGGTATCCATC-3’ and 5’- GCCACAGTTCTCAAAGCACA-3’; VCAM-1: 5’- ACTGTTGACATCTCCCCTGG-3’ and 5’- AGTGTGGATGTAGCCCCTTC-3’; collagen I: 5'- GCCGCAAAGAGTCTACATGT-3' and 5'- AGGTTTCCACGTCTCACCAT -3'; collagen III: 5'- ATTGCTGGAGTTGGAGGTGA-3' and 5'- TGAGTTCAGGGTGGCAGAAT-3'; GAPDH: 5'- CACAGTCCATGCCATCACTG-3' and 5'- GCATGTCAGATCCACAACGG-3'.
Western blot
Total proteins were extracted from the tissues and protein concentration was qualified by bicinchoninic acid protein assay kit. Proteins were lysed and then used for SDS-PAGE electrophoresis. The proteins were subsequently transferred to polyvinylidene fluoride membranes and blocked with 5% nonfat dry milk at room temperature. Membranes were respectively probed with primary antibodies including NF-κB p65, phospho-NF-κB p65 and GAPDH overnight. Next day, the membranes were washed and respectively incubated with corresponding 2nd antibodies. Following several washes, each image was captured on film. Following development, the images were placed into an automatic image analyzer. Monoclonal GAPDH antibody was used as a loading control.
Statistical analysis
The data are expressed as the means ± standard deviation (SD). One-way analysis of variance was used for comparisons following by LSD (equal variances assumed) or Tamhane’s T2 (equal variances not assumed) using SPSS version 13.0 software. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Hesperetin improves glycometabolism in DM rats
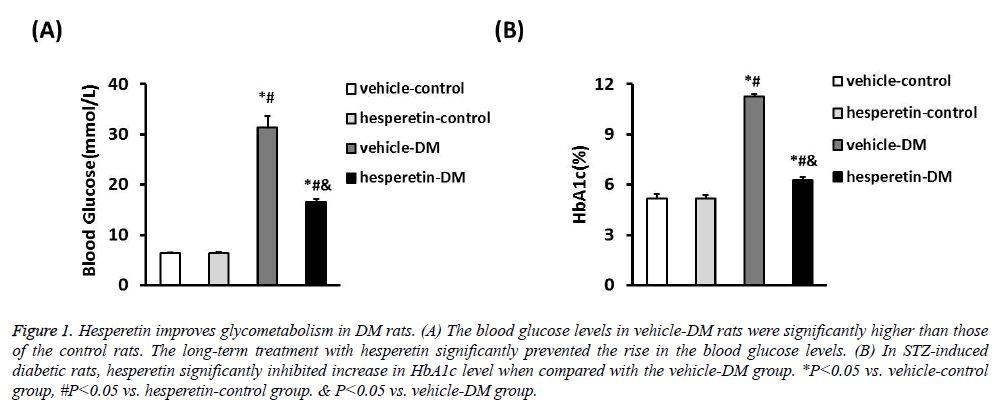
A DM model was established by intraperitoneal injection of STZ in citrate buffer. The vehicle-control and hesperetincontrol rats exhibited stable blood glucose levels. Meanwhile, the blood glucose levels of STZ-induced diabetic rats (31.37 ± 2.34 mmol/L) were significantly higher than those of the control rats. The long-term treatment with hesperetin significantly prevented the rise in the blood glucose levels as compared with the vehicle-DM rats (16.64 ± 0.44) VS. (31.37 ± 2.34) mmol/L). In STZ-induced diabetic rats, hesperetin significantly inhibited increase in HbA1c level after chronic daily drug administration when compared with the vehicle-DM group (6.26 ± 0.21) VS. (11.27 ± 0.15)%) (Figure 1). These results indiacted that administration of hesperetin could improve the disorder status of glycometabolism in DM rats.
Figure 1: Hesperetin improves glycometabolism in DM rats. (A) The blood glucose levels in vehicle-DM rats were significantly higher than those of the control rats. The long-term treatment with hesperetin significantly prevented the rise in the blood glucose levels. (B) In STZ-induced diabetic rats, hesperetin significantly inhibited increase in HbA1c level when compared with the vehicle-DM group. *P<0.05 vs. vehicle-control group, #P<0.05 vs. hesperetin-control group. & P<0.05 vs. vehicle-DM group.
Hesperetin improves cardiac function in DM rats
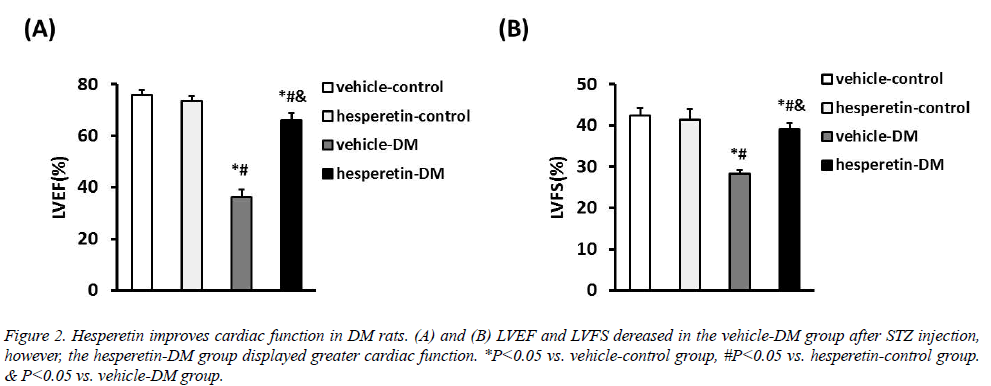
Cardiac function in all animals was assessed with LVEF and LVFS by echocadiography. Compared with the vehicle-DM group, administration of hesperetin improved cardiac function with increase of LVEF ((65.93 ± 2.86) VS. (36.21 ± 2.82)%) and LVFS ((39.11 ± 1.47) VS. (28.26 ± 0.89)%) (Figure 2). These results demonstrated that hesperetin could improve cardiac function in DM rats.
Figure 2: Hesperetin improves cardiac function in DM rats. (A) and (B) LVEF and LVFS dereased in the vehicle-DM group after STZ injection, however, the hesperetin-DM group displayed greater cardiac function. *P<0.05 vs. vehicle-control group, #P<0.05 vs. hesperetin-control group. & P<0.05 vs. vehicle-DM group.
Hesperetin inhibits cardiac inflammation in DM rats
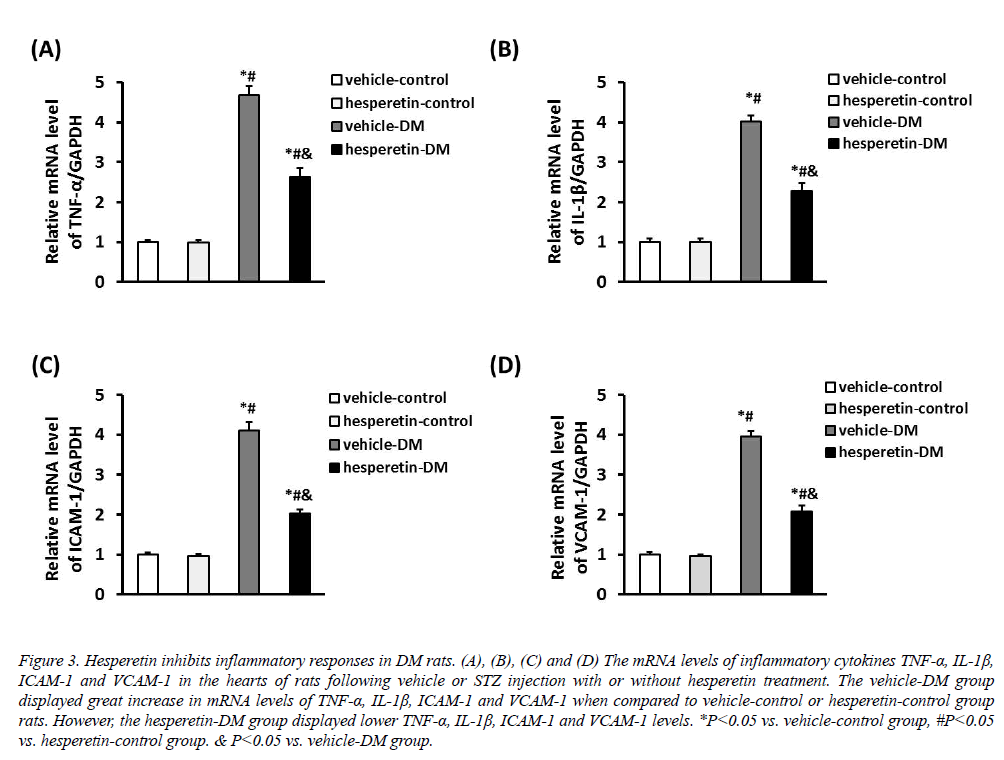
The inflammatory cytokines TNF-α, IL-1β, ICAM-1 and VCAM-1 were increased in rats induced by STZ injection. However, administration of hesperetin significantly reduced the mRNA expression of TNF-α, IL-1β, ICAM-1 and VCAM-1 in the myocardium compared with the vehicle-DM group (Figure 3). These results suggested that hesperetin performed an anti-inflammatory effect on DM rats.
Figure 3: Hesperetin inhibits inflammatory responses in DM rats. (A), (B), (C) and (D) The mRNA levels of inflammatory cytokines TNF-α, IL-1β, ICAM-1 and VCAM-1 in the hearts of rats following vehicle or STZ injection with or without hesperetin treatment. The vehicle-DM group displayed great increase in mRNA levels of TNF-α, IL-1β, ICAM-1 and VCAM-1 when compared to vehicle-control or hesperetin-control group rats. However, the hesperetin-DM group displayed lower TNF-α, IL-1β, ICAM-1 and VCAM-1 levels. *P<0.05 vs. vehicle-control group, #P<0.05 vs. hesperetin-control group. & P<0.05 vs. vehicle-DM group.
Hesperetin protects against cardiac fibrosis in DM rats
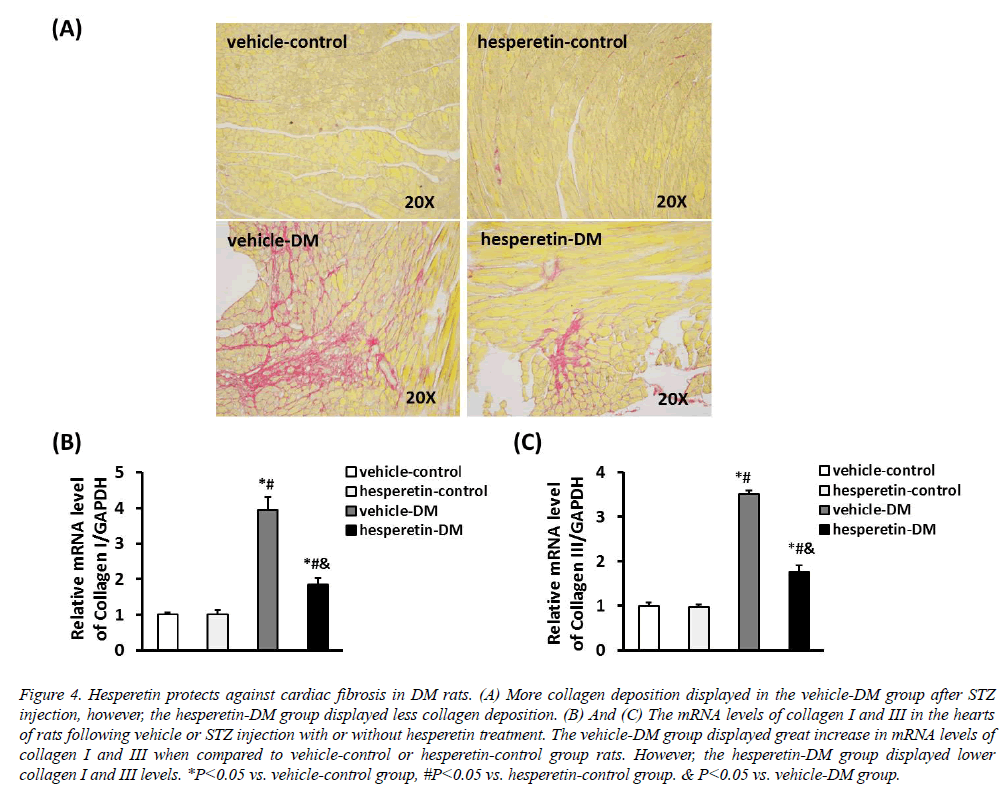
PSR staining showed that there was less collagen deposition in the hesperetin-DM group when compared with the vehicle-DM group (Figure 4). In addition, cardiac fibrosis was estimated by detecting the mRNA levels of collagen I and III. Compared with the vehicle-DM group, administration of hesperetin remarkably reduced the mRNA expression of collagen I and III. These results demonstrated that hesperetin protected from cardiac fibrosis in DM rats.
Figure 4: Hesperetin protects against cardiac fibrosis in DM rats. (A) More collagen deposition displayed in the vehicle-DM group after STZ injection, however, the hesperetin-DM group displayed less collagen deposition. (B) And (C) The mRNA levels of collagen I and III in the hearts of rats following vehicle or STZ injection with or without hesperetin treatment. The vehicle-DM group displayed great increase in mRNA levels of collagen I and III when compared to vehicle-control or hesperetin-control group rats. However, the hesperetin-DM group displayed lower collagen I and III levels. *P<0.05 vs. vehicle-control group, #P<0.05 vs. hesperetin-control group. & P<0.05 vs. vehicle-DM group.
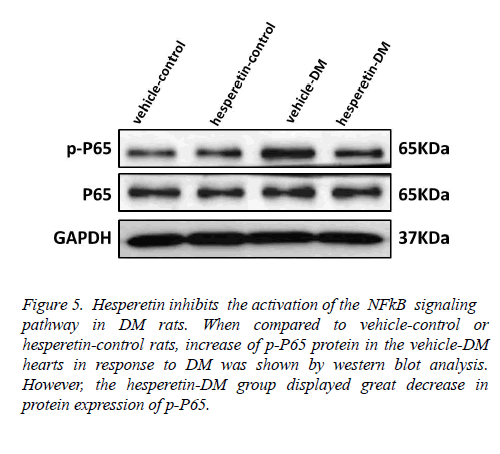
Hesperetin inhibits the activation of the NF-κB signaling pathway in DM rats
In order to clarify the mechanism of hesperetin involved in the inhibition of cardiac inflammation and cardiac fibrosis in DM rats, the role of the NF-κB signaling pathway was detected. Western blot analysis of nuclear and cytoplasmic extracts showed that the level of NF-κB p65 phosphorylation was markedly increased in hearts of the vehicle-DM rats, but NF- κB p65 phosphorylation was reduced in the hesperetin-DM group (Figure 5). These results suggested that administration of hesperetin might perform the antiinflammatory and antifibrosis effects by suppressing NF-κB activation in DM rats.
Figure 5: Hesperetin inhibits the activation of the NFkB signaling pathway in DM rats. When compared to vehicle-control or hesperetin-control rats, increase of p-P65 protein in the vehicle-DM hearts in response to DM was shown by western blot analysis. However, the hesperetin-DM group displayed great decrease in protein expression of p-P65.
Discussion
Diabetic cardiomyopathy (DCM) as an independent clinical disease, its pathogenesis is still not fully elucidated. It is known that a series of pathophysiological processes such as disturbance of carbohydrate metabolism, lipid metabolic disorder, insulin resistance, oxidative stress, inflammatory response, cardiac fibrosis, etc. are involved in its development [1-3]. Studies show that inflammatory response may play an important role in the development of DCM [3,5]. Many inflammatory factors such as macrophage chemotaxis protein-1 (MCP-1), tumor necrosis factor-a (TNF-a), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), interleukin-1β (IL-1β) and interleukin-6 (IL-6) have close relationship with DCM [5,21]. Nuclear factor-κB (NF-κB) is the key factor of the inflammatory signaling pathways, which is involved in transcriptional regulation of a variety of inflammatory factors such as TNF-a and IL-1β [22]. In the present study, after STZ injection, obvious inflammation appeared in myocardial tissue of diabetic rats, characterized by the activation of NF-κB signalling pathway and gene transcription of its downstream, which caused excessive expression of inflammatory factors (TNF-a and IL-1β) and intercellular adhesion factors (ICAM-1 and VCAM-1) detected by their mRNA levels when compared to vehicle-control group. Under the promotion of enhanced inflammation, the expression of collagen I and III within the interstitium and perivascular deposits are increased significantly and disordered arrangement in DM rats which could be found in our present study. Caused by cardiac inflammation and fibrosis, increased myocardial stiffness and impaired ventricular pump function are the main factors to cause left ventricular expansion and heart failure of DM rats, which were indicated by decreased LVEF and LVFS by echocardiography in our study. Therefore, inhibition of inflammatory responses and cardiac fibrosis could attenuate the deterioration of DM and DCM, and looking for a potential drug inhibiting cardiac inflammation and fibrosis to improve cardiac function post DM has important clinical value.
It has reported that traditional Chinese medicine, hesperidin, a flavanone glycoside and the main therapeutic ingredient found in citrus fruit peels, its therapeutic effects mainly consists of anti-oxidative stress, anti-allergy and anti-dermatitis, antibacteria, anti-cancer and genetic toxicity, anti-inflammation, anti-apoptosis and so on [12-14]. In the cardiovascular system, hesperetin has a certain role in dilating blood vessels, reducing blood lipid, lowering permeability of capillary and brittleness of blood vessels confirmed in previous studies [15]. Recently, Deng et al also found that hesperetin could inhibit cardiac remodelling induced by pressure overload in mice [16]. Therefore, hesperetin may play a protective role in the development of DM and DCM. In the present study, it was firstly shown that hesperetin reduced the production of inflammatory cytokines including TNF-α, IL-1β, ICAM-1 and VCAM-1 in myocardium of DM rats. In addition to reduced inflammation reaction, hesperetin also inhibited collagen deposition and the levels of collagen I and III in DM rats’ myocardium. And hesperetin reduced the nuclear localization of nuclear NF-κB p65 and significantly inhibited NF-κB signalling pathway activation. Therefore, our data indicates that the cardio-protective effect of hesperetin may be related to reduced production of inflammatory cytokines and inhibited fibrosis via inhibition of the NF-κB signalling pathway activity. Firstly, hesperetin inhibits the activation of NF-κB signalling pathway, which is a key regulator of the inflammatory response, whose activation could promote inflammation and induce the production of inflammatory cytokines TNF-α, IL-1β and so on. Secondly, hesperetin inhibits cardiac fibrosis induced by inflammation factors, inflammatory cytokines not only can stimulate synthesis of extracellular matrix in cardiac fibroblasts, but also can lead to cardiomyocytes missing induced by cardiac necrosis and apoptosis, which result in cardiac fibrosis [23]. Exhilaratingly, hesperetin can significantly inhibit cardiac inflammatory reaction, protect myocardial tissue from exposure to excessive expression of inflammatory cytokines, and thus eliminate the inflammation factor effect on promoting fibrosis and relieve the degree of cardiac fibrosis of DM rats.
In conclusion, hesperetin therapy could inhibit excessive activation of NF-κB signalling pathway, relieve cardiac inflammation and fibrosis of diabetic rats, and improve cardiac function in diabetic rats, which may provide a basis for a new therapeutic application of hesperetin in the prevention of cardiac remodeling and improving cardiac function following DM and DCM.
Acknowledgements
This work was supported by grant from the National Natural Science Foundation of China (NO.81370872).
References
- Zhang H, Xiong Z, Wang J, Zhang S, Lei L, Yang L and Zhang Z. Glucagon like peptide1 protects cardiomyocytes from advanced oxidation protein product induced apoptosis via the PI3K/Akt/Bad signaling pathway. Mol Med Rep 2016; 13: 1593-1601.
- Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 2016; 12: 144-153.
- Wang ZQ, Chen MT, Zhang R, Zhang Y, Li W, Li YG. Docosahexaenoic acid attenuates doxorubicin-induced cytotoxicity and inflammation by suppressing NF-kappaB/iNOS/NO signalling pathway activation in H9C2 cardiac cells. J Cardiovasc Pharmacol 2016; 67: 283-289.
- Liu Y, Zhang J. Nox2 contribute to cardiac fibrosis in diabetic cardiomyopathy in a transforming growth factor-beta dependent manner. Int J Clin Exp Pathol 2015; 8: 10908-10914.
- Gupta SK, Dongare S, Mathur R, Mohanty IR, Srivastava S, Mathur S, Nag TC. Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol Cell Biochem 2015; 408: 63-72.
- Khanra R, Dewanjee S, T KD, Sahu R, Gangopadhyay M, De Feo V, Zia-Ul-Haq M. Abroma augusta L. (Malvaceae) leaf extract attenuates diabetes induced nephropathy and cardiomyopathy via inhibition of oxidative stress and inflammatory response. J Transl Med 2015; 13: 6.
- Chang W, Zhang M, Meng Z, Yu Y, Yao F, Hatch GM, Chen L. Berberine treatment prevents cardiac dysfunction and remodeling through activation of 5'-adenosine monophosphate-activated protein kinase in type 2 diabetic rats and in palmitate-induced hypertrophic H9c2 cells. Eur J Pharmacol 2015; 769: 55-63.
- Li X, Du N, Zhang Q, Li J, Chen X, Liu X, Hu Y, Qin W, Shen N, Xu C, Fang Z, Wei Y, Wang R, Du Z, Zhang Y, Lu Y. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis 2014; 5: e1479.
- Suzuki H, Kayama Y, Sakamoto M, Iuchi H, Shimizu I, Yoshino T, Katoh D, Nagoshi T, Tojo K, Minamino T, Yoshimura M, Utsunomiya K. Arachidonate 12/15-lipoxygenase-induced inflammation and oxidative stress are involved in the development of diabetic cardiomyopathy. Diabetes 2015; 64: 618-630.
- Luo B, Li B, Wang W, Liu X, Liu X, Xia Y, Zhang C, Zhang Y, Zhang M, An F. Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model. Cardiovasc Drugs Ther 2014; 28: 33-43.
- Wen HL, Liang ZS, Zhang R, Yang K. Anti-inflammatory effects of triptolide improve left ventricular function in a rat model of diabetic cardiomyopathy. Cardiovasc Diabetol 2013; 12: 50.
- Wolfram J, Scott B, Boom K, Shen J, Borsoi C, Suri K, Grande R, Fresta M, Celia C, Zhao Y, Shen H, Ferrari M. Hesperetin liposomes for cancer therapy. Curr Drug Deliv 2015.
- Hoang TK, Huynh TK, Nguyen TD. Synthesis, characterization, anti-inflammatory and anti-proliferative activity against MCF-7 cells of O-alkyl and O-acyl flavonoid derivatives. Bioorg Chem 2015; 63: 45-52.
- Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci 2015; 124: 64-74.
- Liu Y, Niu L, Cui L, Hou X, Li J, Zhang X, Zhang M. Hesperetin inhibits rat coronary constriction by inhibiting Ca(2+) influx and enhancing voltage-gated K(+) channel currents of the myocytes. Eur J Pharmacol 2014; 735: 193-201.
- Deng W, Jiang D, Fang Y, Zhou H, Cheng Z, Lin Y, Zhang R, Zhang J, Pu P, Liu Y, Bian Z, Tang Q. Hesperetin protects against cardiac remodelling induced by pressure overload in mice. J Mol Histol 2013; 44: 575-585.
- Van Linthout S, Spillmann F, Riad A, Trimpert C, Lievens J, Meloni M, Escher F, Filenberg E, Demir O, Li J, Shakibaei M, Schimke I, Staudt A, Felix SB, Schultheiss HP, De Geest B, Tschope C. Human apolipoprotein A-I gene transfer reduces the development of experimental diabetic cardiomyopathy. Circulation 2008; 117: 1563-1573.
- Westermann D, Van Linthout S, Dhayat S, Dhayat N, Escher F, Bucker-Gartner C, Spillmann F, Noutsias M, Riad A, Schultheiss HP, Tschope C. Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes 2007; 56: 1834-1841.
- Jiang DS, Li L, Huang L, Gong J, Xia H, Liu X, Wan N, Wei X, Zhu X, Chen Y, Chen X, Zhang XD, Li H. Interferon regulatory factor 1 is required for cardiac remodeling in response to pressure overload. Hypertension 2014; 64: 77-86.
- Jiang DS, Wei X, Zhang XF, Liu Y, Zhang Y, Chen K, Gao L, Zhou H, Zhu XH, Liu PP, Bond Lau W, Ma X, Zou Y, Zhang XD, Fan GC, Li H. IRF8 suppresses pathological cardiac remodelling by inhibiting calcineurin signalling. Nat Commun 2014; 5: 3303.
- Tillquist MN, Maddox TM. Update on diabetic cardiomyopathy: inches forward, miles to go. Curr Diab Rep 2012; 12: 305-313.
- Volz HC, Seidel C, Laohachewin D, Kaya Z, Muller OJ, Pleger ST, Lasitschka F, Bianchi ME, Remppis A, Bierhaus A, Katus HA, Andrassy M. HMGB1: the missing link between diabetes mellitus and heart failure. Basic Res Cardiol 2010; 105: 805-820.
- Turner NA. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J Mol Cell Cardiol 2015.