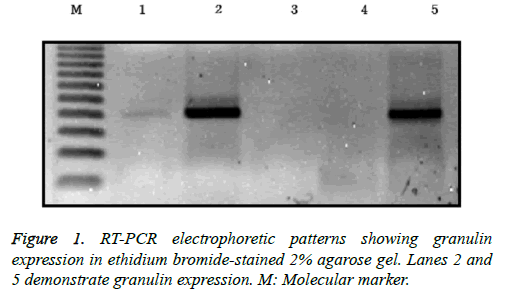
ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 12
Expression of the growth factor granulin in patients with brain tumors: Relevance to prognosis
Je Il Ryu, Myung Hoon Han, Jin Hwan Cheong, Jae Min Kim and Choong Hyun Kim*
Department of Neurosurgery, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri 471-701, Republic of Korea
- *Corresponding Author:
- Choong Hyun Kim
Department of Neurosurgery
Hanyang University Guri Hospital, Republic of Korea
Accepted on May 05, 2017
Granulin is a cysteine-rich polypeptide belonging to a family of growth factors that mediate cell cycle progression and motility. Granulin is expressed in several types of brain tumors. However, its clinical significance in terms of patient outcome has not been determined. This study was conducted to investigate the relationships between granulin expression, clinical characteristics, and prognosis in patients with brain tumors. Tumor tissues were obtained from 295 patients whose brain tumors were surgically resected, and the expression of granulin was assessed by reverse transcriptase polymerase chain reaction (RT-PCR). The tumors were classified pathologically according to World Health Organization (WHO) criteria into non-aggressive (WHO grades I and II) or aggressive (WHO grades III and IV and metastatic) tumors, and the prognostic implications of granulin expression were compared between the two groups. Among the 295 tumor specimens, 107 exhibited positive expression of granulin. For both groups, the mortality rate was significantly different between granulin-positive and -negative tumors (p<0.05). By Kaplan-Meier analysis, the survival rate of patients with granulin-positive tumors was lower than that of patients with negative tumors (p=0.028), and the rate of recurrence was higher in patients with positive tumors than in those with negative tumors (p=0.026). This study suggests that granulin is expressed in various brain tumors and that its expression is correlated with patient prognosis. Granulin may therefore be a novel target for the management of intracranial tumors.
Keywords
Granulin, Brain tumor, Mortality, Prognosis, Survival.s
Introduction
Although brain tumors account for only a small proportion of all cancers, they have higher mortality rates than cancers of other organs. Among studies investigating the molecular mechanisms of brain tumor development, there has been a recent emphasis on the regulation of growth factors and mutations in growth factor signaling pathways [1-4]. Overexpression of various growth factors and related tyrosine kinase receptors is associated with the occurrence of brain tumors. Among these molecules, which include epidermal growth factor (EGF), transforming growth factor (TGF), and epidermal growth factor receptor (EGFR, a common receptor for EGF and TGF), as well as platelet-derived growth factor (PDGF)- and -B and their receptors (PDGFR-A and -B), granulin shares particular similarities with EGF [4,5]. Granulin comprises a family of secreted, glycosylated peptides. Granulin is cleaved from a single precursor protein with 7.5 repeats of a conserved 12-cysteine granulin/epithelin sequence. Cleavage of the signal peptide produces mature granulin, which can be further cleaved into a variety of active, 6 kDa peptides [2,6-10]. These smaller cleavage products are named granulin A, B, C, and D. Granulin is biologically active in various tissues of the body, where it controls cell growth and survival.Generally, a high level of granulin is observed in rapidly proliferating cells such as those of the skin, gastrointestinal tract, kidney, and immune system. Granulin is also involved in tissue repair and tumorigenesis. Although it appears to play a significant function in the survival of nerve cells, the role of granulin in the brain has not been well established [5,6]. Several studies report an association between granulin and frontotemporal dementia. Granulin expression has been assessed in breast cancer, prostate cancer, ovarian cancer, and liver cancer [7,11-16], but it has rarely been investigated in brain tumors beyond glial tumors and meningiomas. In the present study, the expression of granulin was assessed in surgically resected brain tumors. In addition, the clinical implications of granulin expression were investigated, including its relationship with prognosis in patients with brain tumors.
Materials and Methods
Clinical materials and data collection
The medical records of 295 patients with pathologically confirmed brain tumors that were treated by surgical resection between 2001 and 2010 at Hanyang medical center were retrospectively analyzed. Tumor tissues collected from each patient at the time of surgery were immediately frozen and stored at -70°C until experimental processing. Patient surgical records, pathological reports, and radiologic records, including pre- and post-operative computed tomography (CT) and magnetic resonance imaging (MRI) scans, were analyzed to investigate functional outcomes and rates of mortality and tumor recurrence. The study protocol and all related materials were approved by the Institutional Review Board of Hanyang medical center.
Reverse transcriptase polymerase chain reaction (RTPCR)
RNA was extracted from frozen brain tumor tissues according to the manufacturer’s protocol. Tumor tissues were washed three times with phosphate-buffered saline (PBS) and then minced into a powder using a mortar and grinder in liquid nitrogen, after which 1 ml of TRIzol containing 250 μg of glycogen was added. The ground brain tumor tissues were separated and homogenized in 175 μl of RNA lysis buffer containing a 1:50 dilution of ß-mercaptoethanol (BME) until no visible pieces remained. Homogenates were prepared by adding 350 μl of RNA dilution buffer and mixing by inversion 3-4 times. The homogenates were centrifuged for 10 min at 12,000 xg at 4°C, and the supernatants were harvested into fresh tubes. Next, 200 μl of 95% ethanol was added, and the cleared lysate was mixed by pipetting 3-4 times. The resulting mixture was then transferred to a spin column and centrifuged at 12,000 xg for 1 min. The eluent was then discarded, 600 μl of an RNA wash solution containing 95% ethanol was added, and the samples were centrifuged at 12,000 xg for 1 min, after which the eluent was again discarded. The bound material in the column was treated with 50 μl of mixture containing 40 μl of yellow core buffer, 5 μl of 0.09 M MnCl2, and 5 μl of DNase I at room temperature for 15 min. Next, 200 μl of a mixture of 20 ml of 95% ethanol and 5.3 ml of DNase stop solution was added, and the spin column was centrifuged at 12,000 xg for 1 min. After adding 600 μl of SV RNA wash solution, the mixture was centrifuged for 1 min and an additional 250 μl of SV RNA wash solution was added. After centrifugation for 2 min, the spin column was moved to an elution tube. After adding 100 μl of nuclease-free water to the membrane, the column was centrifuged for 1 min and the eluted RNA solution was stored at -70°C. For reverse transcription, 25 μl of Access Quick Master mix 2X (Promega, Madison, WI, USA), 1 μl of upstream primer (5’-TCC ACG TGC TGT GTT ATG GT-3’), 1 μl of downstream primer (5’- CTG CCC TGT TAG TCC TCT GG-3’), 5 μg of RNA template, and nuclease-free water were added to a final volume of 50 μl. Avian myeloblastosis virus (AMV) reverse transcriptase (1 μl) was added and mixed by careful vortexing. Reaction tubes were incubated at 45°C for 45 min. PCR reactions were conducted using 3 μl of the reverse transcription reaction product. Thirty-five cycles of touchdown PCR were carried out according to the manufacturer’s protocol. Each cycle consisted of denaturation at 95°C for 3 min, a one degree per-cycle decrease to a final annealing temperature of 55°C for 1 min, and an extension reaction at 72°C for 6 min, followed by completion of the reaction at 4°C overnight. The resulting PCR products were stored at -20°C. A reaction without an RNA template was used as a negative control. After mixing 8 μl of PCR product and 2 μl of loading buffer, mixtures were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and visualized by ultraviolet illumination (Figure 1).
Statistical analysis
Baseline patient data are presented as the mean ± standard deviation. Discrete variables are expressed as counts (percentages). The Chi-square test for dichotomous variables and the student’s t-test for continuous variables were used to assess clinical differences between patients with granulinnegative and -positive tumors. Kaplan–Meier analysis was performed after censoring patients who were lost to follow-up in order to evaluate mortality and recurrence in the aggressive and non-aggressive tumor groups. Differences between the granulin-negative and -positive groups were compared using a log-rank test. Statistical analyses were performed using R version 3.1.2 and SPSS for Windows, version 22.0 (IBM, Chicago, IL, USA). Values of p<0.05 were considered statistically significant.
Results
Clinical characteristics
The mean patient age was 47.9 ± 16.9 years (range, 3.5 to 82 years). Of the patients, 177 were male and 118 were female. Granulin expression was observed in 107 patients, who had a mean age of 45.5 ± 17.9 years. Among patients with granulinpositive tumors, 64 were men and 43 were women. The mortality and tumor recurrence rates of all patients were 17.6% and 26.4%, respectively. Granulin expression did not significantly differ according to age or sex. The brain tumors were classified pathologically according to 2007 World Health Organization (WHO) criteria [17]. Tumors were divided into two pathological groups, a non-aggressive group (WHO grades I and II) and an aggressive group (WHO grades III and IV, and metastatic tumors) (Table 1).
| Pathological types | No. of patients (%) | Subgroup |
|---|---|---|
| Astrocytic tumors | 42 (14.2) | |
| Glioblastoma | 20 (6.7) | A |
| Anaplastic astrocytoma | 16 (5.4) | A |
| Diffuse astrocytoma | 1 (0.3) | NA |
| Pilocytic astrocytoma | 5 (1.7) | NA |
| Meningioma | 75 (25.4) | NA |
| Pituitary adenoma | 60 (20.3) | NA |
| Schwannoma | 36 (12.2) | NA |
| Craniopharyngioma | 8 (2.7) | NA |
| Malignant meningioma | 2 (0.7) | A |
| Anaplastic ependymoma | 3 (1.0) | A |
| Malignant lymphoma | 3 (1.0) | A |
| Ependymoma | 2 (0.7) | NA |
| Choroid plexus papilloma | 2 (0.7) | NA |
| Anaplastic oligodendroglioma | 4 (1.4) | A |
| Sarcoma | 3 (1.0) | A |
| Metastatic tumor | 35 (11.9) | A |
| Other | 20 (6.8) |
NA: Non-aggressive group; A: Aggressive group.
Table 1. Summary of pathological findings of all patients.
Granulin expression and pathologic types of brain tumors
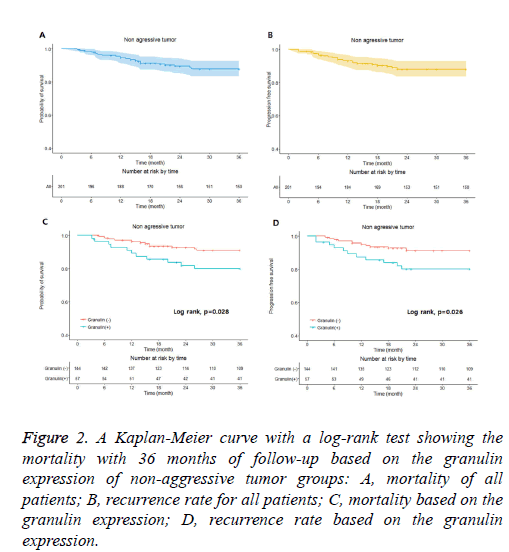
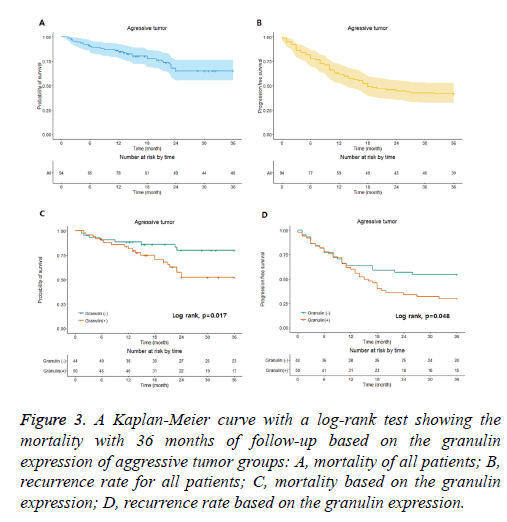
The non-aggressive tumor group consisted of 201 patients with a mean age of 48.3 ± 16.2 years, of which 121 were male and 80 were female (Table 2). Within the non-aggressive tumor group, positive granulin expression was observed in specimens from 57 patients, including 35 men and 22 women with a mean age of 45.8 ± 16.8 years. The mortality of patients with granulin-positive tumors was 19.3%, which was significantly higher than that of patients with granulin-negative tumors (8.3%; p=0.046). However, the rate of tumor recurrence in patients with granulin-positive tumors, which was 22.9%, was not significantly different from that in patients with granulinnegative tumors (12.5%; p=0.057) (Table 2). There were 94 patients in the aggressive tumor group, 56 males and 38 females with a mean age of 46.9 ± 18.5 years (Table 3). In the aggressive group, granulin expression was observed in 50 tumors from 29 men and 21 women with a mean age of 45.2 ± 19.2 years (Figure 3 and Table 3). The mortality and tumor recurrence rates of patients with granulin-positive tumors were 42% and 70%, respectively, which were significantly less than those of patients with granulin-negative tumors (p=0.023 for mortality and p=0.028 for tumor recurrence) (Table 3).
| Variable | Granulin (-) (N = 144) | Granulin (+) (N = 57) | Total (N = 201) | p-value |
|---|---|---|---|---|
| Sex | 0.952* | |||
| Female | 58 (40.3%) | 22 (38.6%) | 80 (39.8%) | |
| Male | 86 (59.7%) | 35 (61.4%) | 121 (60.2%) | |
| Age (years) | 49.3 ± 15.9 | 45.8 ± 16.8 | 48.3 ± 16.2 | 0.162* |
| Mortality | 0.046 | |||
| No | 132 (91.7%) | 46 (80.0%) | 178 (88.6%) | |
| Yes | 12 (8.3%) | 11 (19.3%) | 23 (11.4%) | |
| Recurrence | 0.057* | |||
| No | 126 (87.5%) | 44 (77.1%) | 170 (84.5%) | |
| Yes | 18 (12.5%) | 13 (22.9%) | 31 (15.5%) |
Data are presented as the mean ± standard deviation. *No significant difference.
Table 2. Results of univariate analysis of granulin expression in patients with non-aggressive brain tumors.
| Variable | Granulin (-) (N = 44) | Granulin (+) (N = 50) | Total (N = 94) | p-value |
|---|---|---|---|---|
| Sex | 0.904* | |||
| Female | 17 (38.6%) | 21 (42.0%) | 38 (40.4%) | |
| Male | 27 (61.4%) | 29 (58.0%) | 56 (59.6%) | |
| Age | 48.9 ± 17.7 | 45.2 ± 19.2 | 46.9 ± 18.5 | 0.330* |
| Mortality | 0.023 | |||
| No | 36 (81.8%) | 29 (58.0%) | 65 (69.1%) | |
| Yes | 8 (18.2%) | 21 (42.0%) | 29 (30.9%) | |
| Recurrence | 0.028 | |||
| No | 24 (54.5%) | 15 (30.0%) | 39 (41.5%) | |
| Yes | 20 (45.5%) | 35 (70.0%) | 55 (58.5%) |
Data are presented as the mean ± standard deviation. *No significant difference.
Table 3. Results of univariate analysis of granulin expression in patients with aggressive brain tumors.
Figure 2: A Kaplan-Meier curve with a log-rank test showing the mortality with 36 months of follow-up based on the granulin expression of non-aggressive tumor groups: A, mortality of all patients; B, recurrence rate for all patients; C, mortality based on the granulin expression; D, recurrence rate based on the granulin expression.
Figure 3: A Kaplan-Meier curve with a log-rank test showing the mortality with 36 months of follow-up based on the granulin expression of aggressive tumor groups: A, mortality of all patients; B, recurrence rate for all patients; C, mortality based on the granulin expression; D, recurrence rate based on the granulin expression.
Correlation between granulin expression and prognosis
In the non-aggressive tumor group, the overall survival rate of patients with granulin-positive tumors was significantly worse than that of patients with granulin-negative tumors (Table 2). However, the expression of granulin was not statistically significantly correlated with tumor recurrence. In the aggressive tumor group, the overall mortality and tumor recurrence rates of patients with granulin-positive tumors were significantly worse than those of patients with granulinnegative tumors. These results indicate that the expression of granulin influences both mortality and tumor recurrence in patients with aggressive brain tumors, whereas it influences only the survival rate in patients with non-aggressive tumors. According to a Kaplan–Meier analysis conducted after a mean follow-up of 36 months, in the non-aggressive group, patients with granulin-positive tumors showed lower survival and recurrence rates than patients with granulin-negative tumors (p=0.028 and p=0.026 by log-rank test, respectively) (Figure 2). In the aggressive group, the survival and recurrence rates were lower in patients with granulin-positive tumors than in those with granulin-negative tumors (p=0.017 and p=0.048 by log-rank test, respectively) (Figure 3).
Discussion
Many biomarkers have been used in the diagnosis and treatment of brain tumors. These biomarkers can be detected in body tissues including blood, urine, saliva, and cerebrospinal fluid (CSF), and are used to diagnose disease, determine severity, and establish patient prognosis. Research into tumor biomarkers has recently increased as progress has been made in the fields of proteomics and gene expression analysis [7,18]. Granulins are a family of cysteine-rich polypeptides with the ability to modulate cell growth. Proteins belonging to this family can act as either inhibitors or stimulators of cell growth, and in some cases may have dual activities. Furthermore, they play crucial roles in normal tissue development, wound healing, and tumorigenesis [6,7,10,19,20]. The granulins modulate cell cycle progression and cell motility, and have mitogenic activities in human epithelial and mesenchymal cells. Granulin recognizes and captures DNA derived from external bacteria or viruses and signals to various immune cells to aid in the effective destruction of infected cells [10,21]. Four isoforms of granulin (A, B, C, and D) have been isolated from human inflammatory cells, among which granulin A is the most well-studied. Partial terminal amino acid sequences have been obtained for granulins B, C, and D, which indicate that all four human granulins are closely related. In addition, a fifth granulin, granulin F, has recently been obtained from urine [22].
Many recent studies show that autosomal dominant mutations in the gene encoding granulin are correlated with frontotemporal dementia as well as the development of various types of tumors [7,11,23]. Frontotemporal degeneration is a common cause of early-onset neurodegenerative dementia. Granulin-related frontotemporal dementia is a progressive cerebral disorder that can affect behavior, movement, and language. The gradual loss of neurons resulting from granulin mutations occurs primarily in the frontal and temporal lobes, leading to the aforementioned symptoms [11,23-25]. Overexpression of granulin has also been reported in tumors, mainly in breast carcinoma, where it is known to stimulate the proliferation and survival of cancer cells as well as promote resistance to anti-cancer drugs including tamoxifen and doxorubicin [12,15,16,20,26]. In addition, granulin is overexpressed in ovarian cancer and in cancers of reproductive tissues such as the epididymis, placenta, and prostate [7,13]. In hepatomas, elevated expression of mitochondrial defect gene signatures is associated with decreased survival rates, and NUPR1, one such mitochondrial defect-related gene, directly induces the expression of granulin, thereby contributing to the malignant progression of hepatomas [14,27]. In meningiomas, granulin expression correlates with tumor size and peritumoral brain edema. Furthermore, a 2.1 kb granulin mRNA is expressed predominantly in glial tumors, but is absent in normal brain tissue [8,28]. Granulin shows qualitative similarities to the EGF/TGF system. For example, EGFR and granulin are both located on chromosome 17 [4,5,23]. Mutations caused by the overexpression and hyperactivity of EGFR are associated with glioblastomas, and thus serve as targets of various anti-cancer drugs [29]. In addition, an association between EGFR mutations and the prognosis and chemosensitivity of lung tumors was also recently reported [30]. Granulin expression in human gliomas is also highly correlated with increased malignancy and tumor progression [8]. Together, these findings suggest that the overexpression of granulin as well as mutations in granulin genes are significantly associated with the pathogenesis and malignant progression of brain tumors.
Glioblastoma is the most common primary brain tumor in adults, and predominantly affects elderly persons [17]. Despite treatment options including surgical resection, aggressive radiotherapy, and chemotherapy, the median survival of patients with glioblastoma is approximately 14.6 months [4]. In the present study, granulin expression was especially high in aggressive tumors, and was highly correlated with patient mortality and tumor recurrence. Likewise, patient survival and disease recurrence were significantly different between patients with granulin-positive and -negative tumors. Together, these findings suggest that targeting granulin may represent a novel therapeutic strategy for patients with brain tumors.
This study has several inherent limitations. Because this was a retrospective study, we did not investigate the behavior of granulin after surgical removal of intracranial tumors. Prospective studies may be required to define the postoperative behavior of granulin expression in intracranial tumors. In addition, patients in this study were managed using diverse therapeutic modalities, including chemotherapy and radiotherapy. As therapeutic outcome is different depending on the specific modalities used, even for the same pathological types of tumors, additional variables should be considered when evaluating the effects of granulin expression. Diverse treatment and follow-up protocols may have significantly influenced patient mortality and recurrence. Thus, further studies should be performed in patients with pathologically homogeneous brain tumors and should include analysis of long-term outcomes.
Conclusion
Granulin expression was confirmed in diverse intracranial tumors and was associated with the prognosis of both nonaggressive and aggressive tumors. These results suggest that granulin may be a potential prognostic indicator and therapeutic target in aggressive brain tumors.
Acknowledgements
English grammar was revised by Bioedit Ltd.
References
- Boudreau CR, Liau LM. Molecular characterization of brain tumors. Clin Neurosurg 2004; 51: 81-90.
- Cross M, Dexter TM. Growth factors in development, transformation, and tumorigenesis. Cell 1991; 64: 271-280.
- Markert JM, Fuller CM, Gillespie GY, Bubien JK, McLean LA, Hong RL, Lee K, Gullans SR, Mapstone TB, Benos DJ. Differential gene expression profiling in human brain tumors. Physiol Genomics 2001; 5: 21-33.
- Nagane M, Huang HJ, Cavenee WK. Advances in the molecular genetics of gliomas. Curr Opin Oncol 1997; 9: 215-222.
- Louis DN, Gusella JF. A tiger behind many doors: multiple genetic pathways to malignant glioma. Trends Genet 1995; 11: 412-415.
- Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun 1990; 173: 1161-1168.
- Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays 2009; 31: 1245-1254.
- Liau LM, Lallone RL, Seitz RS, Buznikov A, Gregg JP, Kornblum HI. Identification of a human glioma-associated growth factor gene, granulin, using differential immuno-absorption. Cancer Res 2000; 60: 1353-1360.
- Maris C, D'Haene N, Trépant AL, Le Mercier M, Sauvage S, Allard J, Rorive S, Demetter P, Decaestecker C, Salmon I. IGF-IR: a new prognostic biomarker for human glioblastoma. Br J Cancer 2015; 113: 729-737.
- Park B, Buti L, Lee S, Matsuwaki T, Spooner E, Brinkmann MM, Nishihara M, Ploegh HL. Granulin is a soluble cofactor for toll-like receptor 9 signaling. Immunity 2011; 34: 505-513.
- Alberici A, Archetti S, Pilotto A, Premi E, Cosseddu M, Bianchetti A. Results from a pilot study on amiodarone administration in monogenic frontotemporal dementia with granulin mutation. Neurol Sci 2014; 35: 1215-1219.
- Asaka S, Fujimoto T, Akaishi J, Ogawa K, Onda M. Genetic prognostic index influences patient outcome for node-positive breast cancer. Surg Today 2006; 36: 793-801.
- Davidson B, Alejandro E, Florenes VA, Goderstad JM, Risberg B, Kristensen GB. Granulin-epithelin precursor is a novel prognostic marker in epithelial ovarian carcinoma. Cancer 2004; 100: 2139-2147.
- Lee YK, Jee BA, Kwon SM, Yoon YS, Xu WG, Wang HJ. Identification of a mitochondrial defect gene signature reveals NUPR1 as a key regulator of liver cancer progression. Hepatology 2015; 62: 1174-1189.
- Lu R, Serrero G. Stimulation of PC cell-derived growth factor (epithelin/granulin precursor) expression by estradiol in human breast cancer cells. Biochem Biophys Res Commun 1999; 256: 204-207.
- Serrero G. Autocrine growth factor revisited: PC-cell-derived growth factor (progranulin), a critical player in breast cancer tumorigenesis. Biochem Biophys Res Commun 2003; 308: 409-413.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97-109.
- Hartwell L, Mankoff D, Paulovich A, Ramsey S, Swisher E. Cancer biomarkers: a systems approach. Nat Biotechnol 2006; 24: 905-908.
- Kleinberger G, Capell A, Haass C, Van Broeckhoven C. Mechanisms of granulin deficiency: lessons from cellular and animal models. Mol Neurobiol 2013; 47: 337-360.
- Yeh JE, Kreimer S, Walker SR, Emori MM, Krystal H, Richardson A. Granulin, a novel STAT3-interacting protein, enhances STAT3 transcriptional function and correlates with poorer prognosis in breast cancer. Genes Cancer 2015; 6: 153-168.
- Wu H, Siegel RM. Medicine. Progranulin resolves inflammation. Science 2011; 332: 427-428.
- Sparro G, Galdenzi G, Eleuteri AM, Angeletti M, Schroeder W, Fioretti E. Isolation and N-terminal sequence of multiple forms of granulins in human urine. Protein Expr Purif 1997; 10: 169-174.
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006; 442: 916-919.
- Premi E, Grassi M, Gazzina S, Paghera B, Pepe D, Archetti S. The neuroimaging signature of frontotemporal lobar degeneration associated with Granulin mutations: an effective connectivity study. J Nucl Med 2013; 54: 1066-1071.
- Premi E, Cauda F, Costa T, Diano M, Gazzina S, Gualeni V. Looking for Neuroimaging Markers in Frontotemporal Lobar Degeneration Clinical Trials: A Multi-Voxel Pattern Analysis Study in Granulin Disease. J Alzheimers Dis 2016; 51: 249-262.
- Tangkeangsirisin W, Serrero G. PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis 2004; 25: 1587-1592.
- Yung MK, Lo KW, Yip CW, Chung GT, Tong CY, Cheung PF. Copy number gain of granulin-epithelin precursor (GEP) at chromosome 17q21 associates with overexpression in human liver cancer. BMC Cancer 2015; 15: 264.
- Kim CH, Cheong JH, Kim JM. Correlation of granulin expression in intracranial meningiomas to clinical parameters. Exp Ther Med 2010; 1: 493-496.
- Li X, Wu C, Chen N, Gu H, Yen A, Cao L, Wang E, Wang L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 2016; 7: 33440-33450.
- Suda K, Mitsudomi T. Role of EGFR mutations in lung cancers: prognosis and tumor chemosensitivity. Arch Toxicol 2015; 89: 1227-1240.