ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2008) Volume 19, Issue 1
Establishing Therapeutic Bioequivalence of a Generic Salbutamol (Butalin®) Metered Dose Inhaler to Ventolin
Abdulrahman Al Frayh1*, Abdullah Abba1, Aymen Iskandarani2, Duraid S. Shaker3, Fares Zai-toun4, Layla Shahrabani5, Mohammed Khan6, Moussa Riachy7, Ruwayda Dham3
1King Khalid University Hospital/ KSA
4American University Hospital/ Lebanon
6Al Rashed Aleergy Center/ Kuwait
7Hotel-Dieu De France Hospital/ Lebanon
- *Corresponding Author:
- Abdulrahman Al-Frayh
Department of Pediatrics, College of Medicine, King Saud University
P.O. 2925, Riyadh 11461, Saudi Arabia
E-mail: alfrayh@yahoo.com
Accepted date: February 17 2008
Inhaled beta-adrenergic receptor agonist plays an important role in the management of bronchial asthma especially in the acute setting and still salbutamol (albuterol, USAN) is the most widely prescribed drug in this class. In a randomized, open label, crossover, multicenter study, one and two actuations (100, 200μg) of Ventolin and Butalin inhaler were administered in 4 alternative different conse-quences for patients suffering from mild to moderate asthma for 4 days trial compares forced expiratory volume in the first second (FEV1) at (0, 15, 30, 45 and 60 minutes) and peak expiratory flow (PEF) at (0, 15, 30, 45, 60, 120, 240, 360 minutes) to prove equivalent pharmacodynamics, equipotency and safety. Eighty-nine patients completed the study of which 61.8% were male. There was no signifi-cant difference between Ventolin and Butalin concerning the average of Ln transformed re-cords for the FEV1 and PEF, and the AUFC0-1h for the 100 and 200 μg were (96.89-101.07) and (92.69-104.77) respectively, same for the PEF AUC0-4h that came with the ranges of (99.20-104.24) and (90.25-100.49) respectively. No significant difference was noted between the two products in regard of onset and duration of action, and the same was true when as-sessing products potency. Concluding that both products are clearly equivalent and equipotent with similar safety profile.
Keywords
Salbutamol, generic, mild to moderate asthma, Middle East, pharmacodynamics.
Introduction
Bronchodilator drugs used alone or in conjunction with inhaled corticosteroid or other anti-inflammatory drugs are essential for the management of asthma [1,2]. β2-adrenoceptor agonist considered one of the main effective categories and the short-acting salbutamol has been for long the most widely used bronchodilator. Most interna-tional guidelines recommend the use of beta-adrenergic agonist in the form of metered dose inhaler (MDI) for quick symptomatic relief [3].
Metered dose inhalers (MDIs) containing chlorofluoro-carbon propellants are the dominant delivery system for bronchodilators. Concerns about the depletion of the ozone layer by the catalytic action of the free chlorine radicals from the chlorofluorocarbons had led to legisla-tion calling for the elimination of the chlorofluorocarbon production [4].
Hydrofluoroalkanes, as hydrofluoroalkane-134a, do not contain chlorine and are therefore environmentally friendly alternative for use as propellants in the MDIs. They are known to be safe and efficacious as substitute for chlorofluorocarbon 11/12 with β2agonists like salbu-tamol and have already been licensed for clinical use (Proventil, Airomir).
The incessant increase in the incidence of asthma and the requirement for new products phasing out chlorofluorocarbon (CFC) propellants, added to the need for cost ef-fective medication in relation to the existing treatment, were the key factors for pharmaceutical industries to re-spond to a decade of off-patent availability of effective asthma drugs including salbutamol and encourage the development of novel generic bronchodilator/ inhaler de-vice combinations [5].
Hence, the emergence of generic substitute has led to a pressing need to develop a precise and economical method to evaluate its potency and equivalence to current alternative in order to satisfy both clinician and regulatory authorities. The US Food and Drug Administration con-sidered two inhaled formulations as bioequivalent if the 90% confidence interval (CI) of the relative potency is between 0.67 and 1.50.
Although in vitro comparisons between inhaled products are useful, still they do not reflect or can predict in vivo drug delivery to the site of action. The three principal in vivo methods currently available to study the bioequiva-lence of inhaled medications are radioaerosol drug depo-sition studies, pharmacokinetic studies and comparative pharmacodynamic clinical efficacy studies [6,7].
Radioaerosol studies that used gamma scintigraphy pro-vide useful information about lung deposition but their predictive ability for clinical efficacy of β agonist is proven to be variable [8]. Pharmacokinetic studies on the other hand are of limited value for inhaled medications because the dose administrated is small and the resulting serum concentrations are often too low to assay accu-rately. Furthermore, serum concentrations may not corre-late with the dose delivered to the lung. A urine assay of the salbutamol has been described as example of pharma-cokinetic studies [9], however, there is no enough credi-bility of the bioequivalence data in subjects with asthma.
Clinical pharmacodynamics efficacy studies are ulti-mately the most clinically useful measure of the effec-tiveness of inhaled β agonist. As it is well known that β2 agonists have two distinct pharmacodynamic effects of clinical importance in asthma: bronchodilation which is the most trend adapted in “in vivo” bioequivalence stud-ies [10-13] and prevention of bronchoconstriction caused by direct agent like methacoline [14] or indirect agent like histamine [15] or exercise, cold air or allergen.
Both of these responses are clinically and physiologically distinct and important but demonstration of the bioequiva-lence of bronchoprotective effect is particularly important in patients with mild asthma who have minimal or no air flow obstruction and in whom it might be difficult to compare FEV1 values. Furthermore, there is some rec-ommendation by the British Association of Lung Re-search as to how bronchodilator studies should be con-ducted [6] but none for conducting or analyzing the rela-tive potencies of the bronchoprotective effects. The study corresponds to clinical study phase 4, designed as open, randomized, crossover with 4 sequences, 4 treatments and last for 4 days, multicenter performed at 5 centers in 3 Middle Eastern countries. The treatment was administrated on each of the four separate study visits. At least 24 hours and not more than 3 days were permitted between study visits. The random distribution to assign each patient to a specific therapeutic regime was carried out centrally by Proc Scheme (SAS, version 8.0). Since long, measures of bronchodilator drug efficacy have relied on direct measurement of the induced bronchodila-tation by lung function testing, even though that this method does not allow potency discrimination between drug products, as single dose can result in maximal bron-chodilatation [16].
The forced expiratory volume in the first second (FEV1), which like other spirometeric indices is affected by air-way obstruction, is generally accepted as the most useful measure of airway potency since it is considered to be repeatable index. Peak expiratory flow (PEF) measure-ment is a convenient way of monitoring longer-term drug effects on lung function during clinical trials but still more effort dependent than the relatively prolonged FEV1 and although average changes in FEV1 and PEF are simi-lar during bronchodilatation or constriction, the variability of PEF is greater.
In this study rigorous adherence to standardized spirome-try techniques according to the American Thoracic Soci-ety [17] was fundamental to the accurate use of the above end points and both values were made more reliable by taking the best of at least three attempts, but accuracy was better assured during spirometry where a low forced vital capacity (FVC) reading, if this was inconsistent, indicates a poor effort which can validly discounted.
Butalin® Inhaler (Julphar`s Salbutamol) contains 200 ac-tuations of 100μg Salbutamol Micronized (as sulfate) be-ing the active ingredient, and oleic acid with norflurane as excipients, and contains (HFA 134a) Hydrofluroalkane as propellant, it is Chlorofluorocarbons (CFC) Free.
Material and Methods
The main purpose of the study was to assure the clinical equivalence (effectiveness and safety), equipotence and parallel pharmacodynamics (onset and duration of action) of Butalin Inhaler and Ventolin Inhaler (GlaxoWell-come`s Salbutamol) on asthmatic patients classified ac-cording to the Global Initiative Guidelines (GINA) under step 1, 2 and 3, with both 100 and 200μg dosages.
The study corresponds to clinical study phase 4, designed as open, randomized, crossover with 4 sequences, 4 treatments and last for 4 days, multicenter performed at 5 centers in 3 Middle Eastern countries. The treatment was administrated on each of the four separate study visits. At least 24 hours and not more than 3 days were permitted between study visits. The random distribution to assign each patient to a specific therapeutic regime was carried out centrally by Proc Scheme (SAS, version 8.0).
The dose was delivered via a spacer device (Optichamber, Respironics, USA) that was distributed to all patients foll-owing the criteria of the European Respiratory Society [18]. Spirometry was done at baseline and repeated every 15 minutes for the first hour of the test using Micro-lab3500 (Micromedical, UK) in all study centers. Simi-larly PEF was also recorded during the first hour of the test and then the subject had to measure it hourly for an-other 5 hours duration using hand held peak flow meter (Personal Best, Respironics, USA) that was supplied as study material to all subjects.
Asthmatic patients as defined by the American Thoracic Society and those who could consistently perform repro-ducible spirometry from both sexes, aged 15 to 60 years, with FEV1 ranged between 60-90% of the theoretic value and FEV1/FVC <70% were enrolled after showing a bronchodilatory effect as FEV1 increased by >12% fif-teen minutes post 200μg salbutamol dose, fulfilling all selection criteria, considering not to include patients treated with systemic corticosteroid or ß-adrenergic an-tagonist or been recently on inhaled corticosteroid ther-apy.
The variables that determine the clinical equivalence are the 90% confidence interval for area under curve (AUC) of the effect on 15 minutes interval measured FEV1 ver-sus time between baseline time and 1 hour (AUC0-1h) and the effect on one hour measured PEF between baseline and 6 hours (AUPEC0-6h), and were determined by the liner trapezoid rule, as well as the percentile of the maxi-mum FEV1 and PEF change from baseline (FEV1 and PEFmax%). Pharmacodynamics including onset and dura-tion of action were determined by liner interpolation, con-sidering a 10% change of FEV1 from baseline as onset point and duration of action end when PEF less than 10% from baseline.
Adverse events were illnesses or signs or symptoms that appeared or worsen during of the study. All adverse events, including observed elicited, or volunteered prob-lems, complaints or symptoms, were recorded on the Ad-verse Events Case Report Form. Each adverse event is to be evaluated for date/time of onset, duration, intensity, seriousness and casual relationship with the clinical study material or other factors according to Karch & Casagna. Tolerability was assessed by measuring blood pressure, heart rate and ECG (lead II).
The study was carried out in accordance with Good Clini-cal Practice and the Declaration of Helsinki. All patients gave informed consent. The Ethics Committee of the Ministry of Health, UAE as well as the Ethics Commi-ttees of participating centers approved the protocol.
Results
Demographic data
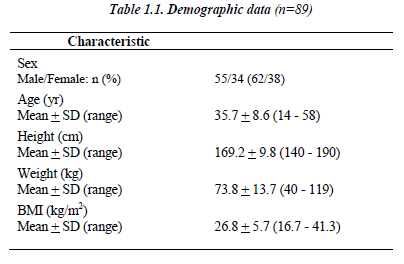
Eighty-nine non-smoking patients (55 males/ 34 females) were enrolled between July 2004 and March 2006 from 5 centers in 3 Middle Eastern countries, with an average age of 35.7 + 8.6 years and 26.8 + 5.7 kg/m2 for BMI (Table 1.1).
Patients suffered from asthma since 8.7 + 8.3 years (1 - 37). Average ideal FEV1 for male was 3.7 + 0.5 (2.3 – 4.7) and for females 2.8 + 0.4 (1.9 – 3.5), while average ideal FVC for males was 4.3 + 0.6 (2.7 – 5.7) and females 3.5 + 0.5 (2.1 – 3.7) using Knudson et al. 19 The FEV1 change percent from the predicted ideal value varies be-tween 54 to 92 with average of 68.2 + 13.3 after taking into consideration the patient’s sex, age and height.
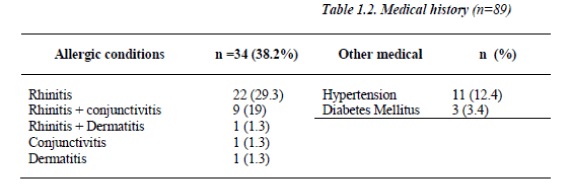
The bronchodilator sensitivity test calculated as percent of change of the FEV1 fifteen minutes post inhalation of 200 μg Salbutamol revealed sensitivity average percentile of 31 + 14.9 and range of (12 - 35). 45.3% (n=34) of the patients had other allergic conditions (Table 1.2).
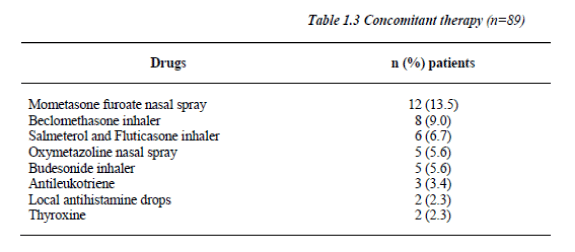
Salbutamol dose was fixed during the study and balanced across visits using the Latin square method. 16.9% of the patients (n=15) were on Mometasone furoate nasal spray (Table 1.3), and compliance for study medications reached 100% with no violations to the study protocol or withdrawal.
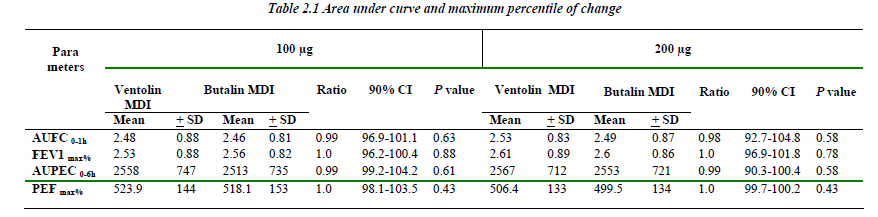
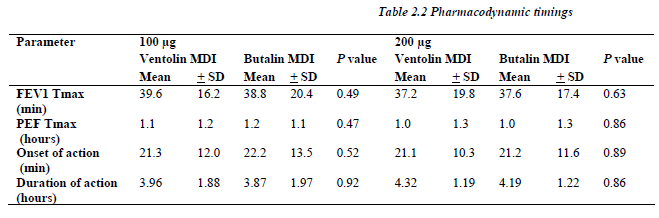
The area under FEV1 and PEF curves versus time for both the 100 and 200μg of Butalin MDI were comparable to the same recorded with Ventolin MDI. Similarly, no significant difference was detected between the two MDIs in concern of the maximum percentile of change for both FEV1 and PEF rate (Table 2.1).
Pharmacodynamic results
Mean baseline of FEV1 natural logarithmic (Ln) trans-formed values were 0.75 + 0.39 for 100μg Butalin day vs. 0.74 + 0.38 for 100μg Ventolin day, while for the 200μg dosage was 0.73 + 0.35 for Butalin day vs. 0.71 + 0.31 for Ventolin day.
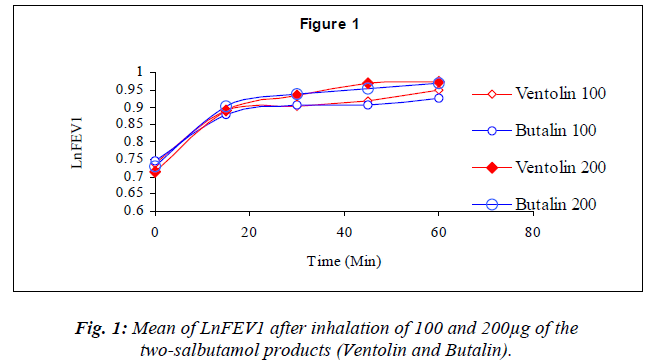
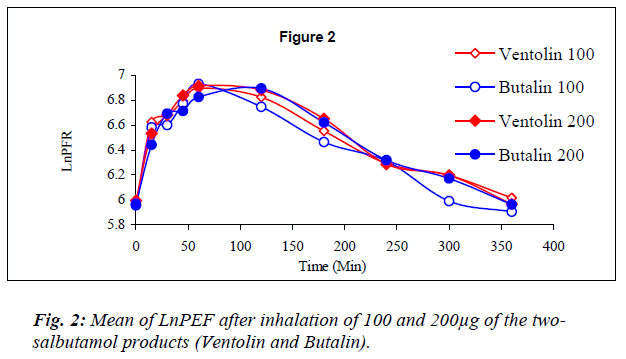
Dose response graphs of Ln FEV1 over the first hour be-tween the two inhalers were superimposed (Fig.1), and the same was demonstrated with six hours Ln PEF (Fig.2) to prove therapeutic equivalency.
Albeit there was no significant difference between the 100 and 200μg FEV1 readings for any of the two MDIs, equi-potency was assessed via comparing the difference of between the two dosages of each over the first hour of the test and the results came insignificant (P> 0.05).
All patients showed significant response (more that 10% change at the FEV1) defined as onset of the bronchodilator effect to both Salbutamol MDIs and for each the 100 and 200μg dosage at almost the same time point (p> 0.05), and the bronchodilatation continue for a like dura-tion between similar dosages of both MDIs (Table 2.2).
Safety results
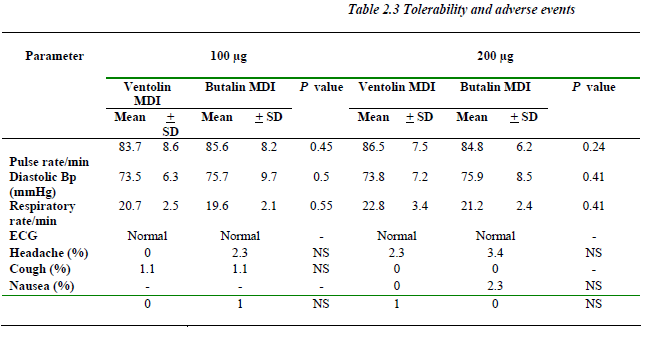
No significant change (P> 0.05) was noticed between baseline and hour one readings concerning vital signs and ECG. Similar incidences of adverse events including hea-dache, cough, nausea and palpitation were recorded for both formulations with similar severity and causal rela-tion. No serious adverse events were reported (Table 2.3).
Discussion
Salbutamol MDI is the most widely prescribed drug in its category [20]. Although the use of chlorofluorocarbons (CFCs) as propellants in MDIs have been permitted under a temporary essential use exemption from Montreal Pro-tocol, 21 pharmaceutical companies have developed al-ternative CFC-free propellant.
Albeit that the patent for Salbutamol expired in 1989, the first generic metered dose inhaler (MDI) preparation of Salbutamol was not approved by the US FDA until late 1995 [22]. This delay resulted primarily from the lack of an acceptable and valid method for establishing in vivo bioequivalence of the generic salbutamol inhaler to the innovator preparation Ventolin.
A number of methodological approaches have been advo-cated for the assessment of generic MDI bioequivalence. The FDA did not consider the plasma pharmacokinetics [23-25] or Gamma scintigraphy methods as reliable re-flection of the relative quantity of drug delivered to the site of action in the lung (the β2 biophase) [26,27].
For this reason, still the clinical studies of inhaled β adrenergic preparation in subjects with asthma that rely on the measurement of the clinically relevant pharmaco-dynamic response of salbutamol are the most creditable to reflect the relative quantity of drug delivered to the ef-fecter compartment in the lung by the generic and the in-novator preparations.
In our study we used 2x2 considering two dosages for the two formulations in 4 different sequences in each two dosages of both formulations are administrated. This does not only provide a more powerful evaluation of the dose response relationship, but also provides an opportunity to prove whether the dose-response curves are parallel of the two formulations.
Results presented here provide assessment of the potency per actuation of the generic salbutamol (Butalin inhaler) relative to that of Ventolin inhaler. Each actuation of the Butalin inhaler is estimated to deliver a quantity of salbu-tamol to the β2 receptors in lung that is equivalent to 1.01 actuations of the Ventolin MDI, with the 90% CI for this estimate 96.9 to 101.1. Stated in another way, this indi-cates that each actuation of Butalin inhaler delivers significantly >96.9% (p<0.05) and significantly <101.1% (p<0.05) as much salbutamol to the lung receptors as does one actuation of Ventolin. The fact that CI lies entirely within the range that the FDA currently accepted provides evidence that Butalin is bioequivalent to Ventolin. The same is true regarding the maximum percentile of bron-chodilation changes, also the onset and total effect dura-tion of one and two actuations of the generic salbutamol analogous to that resulted from Ventolin.
A number of factors may interfere with the delivery and effect of inhaled medications. One of such is the type of spacer device used. Barry and O’Callaghan have demon-strated that the use of two different devices was associ-ated with different amounts of drugs delivered [28,29]. The use of a standard aero chamber in all patients in this study eliminates one such problem. The comparable re-sults obtained may suggest that this factor plays no sig-nificant role since our patients had mild to moderate asthma.
A common problem with this kind of studies response is the failure to show a significant dose-response relation-ship,12 as we found that in spite of the fact that the effi-cacy FEV1 average reading were almost a mirror image between both MDIs for the two dosages but no significant difference in response was recorded between the 100 and 200μg dosages (P>0.05).
The development of safe and comparable generic of inno-vator brand drugs is vital in containing healthcare costs. The cost of generic brands has been found to be much less than the innovator brands in a recent study from Kuwait. [30]. While a study like the current one is important in establishing the efficacy and safety of these brands.
Conclusion
In conclusion and according to FDA Guidance on Bioavailability and Bioequivalence “Bioavailability and Bioequivalence Studies for inhaled Drug Products – Gen-eral Considerations, U.S. Department of Health and Hu-man Services, Food And Drug Administration, Center for Drug Evaluation and Research (CDER)”, the results of the present study demonstrate that the Test product (Buta-lin Inhaler®, Gulf Pharmaceutical Industries/ Julphar) is clinically interchangeable with the Reference product (Ventolin Inhaler®, GlaxoWellcome) with similar phar-macodynamic profile.
Acknowledgments
The authors thank Dr. Iqbal Shadood for her valuable par-ticipation in the study.
References
- National asthma education and prevention program. Expert panel report 2: Guidelines for the diagnosis and management of asthma. NIH, Bethesda, MD; 1997; publication number 97-4051: 31-70.
- British thoracic society guidelines on asthma manage-ment. Thorax 1997; 52: S1-S21.
- Canadian Asthma Consensus Report, 1999 Supplement to Canadian Medical Association Journal 1999; 161 (11 Suppl).
- The Montreal protocol. The Montreal protocol on sub-stances that deplete the ozone layer. Final act (Nairobi: UNEP 1987). Fed reg 1994; 59: 56276-56298.
- Bell J. International development of portable inhalers. J Invest Allergol Clin Immunol 1997; 7: 417-419.
- British Association of Lung Research. Determining equivalence of inhaled medications. Respir Med 1995; 89: 253-261.
- Wong BJO, Hargreave FE. Bioequivalence of metered dose inhaled medication. J Allergy Clin Immunol 1993: 92: 373-379.
- Krishnan Parameswaran. Concepts of establishing clinical bioequivalence of chlorofluorocarbon and hy-drofluoroalkane B agonists. J Allergy Clin Immunol, 1999: 104: 6: S243-5.
- Hindle M, Chrystyn H. Determination of the relative bioavailability of salbutamol to the lung following in-halation. Br J Pharmacol 1992; 34: 311-315.
- Britton J, Hanley SP, Ganett HV, et al. Dose related of salbutamol and ipratropium bromide on airway caliber and reactivity in subjects with asthma. Thorax 1988; 43: 300-305.
- Kleerup EC, Tashkin DP, Cline AC, et al. Cumulative dose response study of non CFC propellant HFA 134a salbutamol sulfate metered dose inhaler in patients with asthma. Chest 1996; 109: 702-707.
- Dockhom R, Venden Burgt JA, Ekholm BP, et al. Clinical equivalence of a novel non-clorofluorocarbon containing salbutamol metered dose inhaler and a con-ventional chlorofluorocarbon inhaler in patients with asthma. J. Allergy Clin Immunol 1995; 96: 50-56.
- Tinkelman DG, Bleecker ER, Ramsdell J, et al. Proven-til HFA and Ventolin have similar safety profiles dur-ing regular use. Chest 1998; 113: 290-396.
- Wong AG, O`Byrne PM, Lindbiadh C, et al. Dose- response protective effect of salbutamol on methacho-line airway responsiveness using pressurized metered dose inhalers and turbuhalers. Can Respir J 1998; 5: 119-123.
- Ahrens RC, Harris JB, Milavetz G, et al. Use of bron-chial provocation with histamine to compare the phar-macodynamic of inhaled albuterol and metaproterenol in patients with asthma. J Allergy Clin Immunol 1987; 79: 876-882.
- H. Buck, M. Parry-Billings. Discriminating measures of bronchodilator drug efficacy and potency. Br J Clin Pharmacol 2001; 52: 245-253.
- American Thoracic Society. Standardization of spi-rometry- update. Am Rev Respir Dis 1987; 136: 1285- 1298.
- Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung vol-umes and forced ventilatory flows. Report of working party of European Community for Steel and Coal: Offi-cial Statement of European Respiratory Society. Eur Respir.J 1993;6 (suppl 16): 5-40.
- Knudson RJ, Lebowitz MD, Holberg CJ, et al. Changes in the normal expiratory flow volume curve with growth and aging. Am Rev. Resp. Dis. 1983; 127: 725-734.
- Top 200 Rx Drugs. In Drug Topic Red Book. Medical Economics Company, NJ 1997; 124
- D’Souza S. The Montreal Protocol and essential use exemptions. J Aerosol Med 1995; 8:: 13 – 17.
- Barbara A, Stewart MD, Richard C, et al. Demonstra-tion of In vivo Bioequivalence of generic albuterol me-tered dose inhaler to Ventolin. Chest 2000; 117:3: 714-721.
- Hindle M, Chrystyn H. Relative bioavailability of sal-butamol to the lung following inhalation using metered dose inhalation method and spacer devices. Thorax 1994; 49: 549-53.
- Hindle M, Newton DA, Chrystyn H. investigation of an optimal inhaler technique with the use of urinary salbu-tamol excretion as a measure of relative bioavailability to the lung, Thorax 1993; 48:607-10.
- Hindle LM, Newton DA, Chystyn H. Dry powder in-halers are bioequivalent to metered dose inhalers: a study using a new urinary albuterol (salbutamol) assay technique. Chest 1995; 107: 629-33.
- Food and Drug Administration. Interim guidance for documentation of In vivo bioequivalence of albuterol aerosols (metered-dose inhalers). Rockville, MD: Divi-sion of bioequivalence. Office of generic drugs, food and drug administration; January 27, 1994; Publication No. 1-27.
- Chystyn H. Standard for bioequivalence of inhaled products. Clin Pharmacokinet 1994; 26:1-6.
- Barry PW, O’Callaghan C. In vitro comparison of the amount of salbutamol available for inhalation from different formulations used with different spacer devices. Eur Respir J 1997; 10: 1345-1346.
- Miller MR, Bright P. Differences in output from corti-costeroid inhaler used with a volumetric spacer. Eur Respir J. 1995; 8: 1637-1638.
- Ball D, Tisocki K, Al Saffar N. Medicine Prices in the State of Kuwait. Report of a Survey of Medicine Prices in Kuwait, March 2005.