ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
Effect of rapid ventricular pacing on cerebral oxygenation in transcatheter aortic valve implantation (TAVI): role of routine near-infrared spectroscopy monitoring
1Department of Anesthesiology, Istanbul Medipol University, Istanbul, Turkey
2Department of Cardiovascular Surgery, Istanbul Medipol University, Istanbul, Turkey
3Department of Cardiology, Istanbul Medipol University, Istanbul, Turkey
- *Corresponding Author:
- Pelin Karaaslan
Department of Anaesthesiology
Medipol Mega Hospital, Istanbul Medipol University, Turkey
Accepted on December 09, 2016
Objective: Transcatheter aortic valve implantation has become an important treatment modality in patients with high risk comorbidities for surgical aortic valve replacement. The objective of this study is to evaluate the cerebral perfusion status using near infrared spectroscopy method especially during the rapid ventricular pacing phase of the transcatheter aortic valve implantation procedure.
Methods: 20 consecutive patients undergoing trans-femoral aortic valve implantation procedure between May 2015 and March 2016 in our institute were retrospectively evaluated. The periprocedural cerebral oxygenation was measured with a near infrared spectroscopy sensor (INVOSTM-5100 C, Medtronic Inc., Minneapolis, MI, USA) located on the forehead of the patients. All hemodynamic data and cerebral near infrared spectroscopy values were recorded before, during and after the procedure with constant time intervals, especially at the time of rapid ventricular pacing and device deployment.
Results: The mean age was 74.4 ± 9.2 years. Male female ratio was 1.8 to 1 (13 males, 7 females). Mean procedure time was 70.2 ± 14.3 minutes. The rapid ventricular pacing included two episodes with a total time for pacing of 22.6 ± 5.1 seconds. There was a statistically significant difference with regard to the heart rate and the cerebral near infrared spectroscopy values (p=0.006 and p=0.02; respectively) in all patients during the rapid ventricular pacing period. The cerebral near infrared spectroscopy values were statistically lower than baseline levels (p<0.001).
Conclusion: This observational study presents the significant decrease of cerebral near infrared spectroscopy values during the rapid ventricular pacing phase of the transcatheter aortic valve implantation procedure. Further studies may reveal cut-off values both for near infrared spectroscopy values and rapid ventricular pacing duration in order to determine a critical cut-off level.
Keywords
Transcatheter aortic valve implantation, Near infrared spectroscopy, Cerebral ischemia, Aortic valve stenosis, Cerebrovascular stroke, Cardiac pacing.
Introduction
Aortic stenosis (AS) is an emerging public health problem with the aging population and it has become the third leading cause of structural heart disease after hypertension and coronary artery disease in industrialized countries [1]. The traditional treatment of AS with surgical aortic valve replacement (SAVR) may be prohibitively risky or even contraindicated in a significant percentage of this patient population due to the advanced age and associated co-morbidities [2]. The balloon aortic valvuloplasty (BAV) was emerged as a less invasive intervention method for high-risk candidates for SAVR with satisfactory short term results in terms of mean aortic valve area and left ventricular ejection fraction [3]. At the beginning of the new century, transcatheter aortic valve implantation (TAVI) had been introduced by the pioneering work of Alain Cribier and it has been nominated as the most exciting development in the last decade in the era of cardiovascular diseases [4,5].
Cerebral injury is probably the most devastating of the nonfatal complications in the postoperative period both for SAVR and TAVI. When the surgically high-risk patient population for TAVI is considered, it’s arguable that these patients have even more risk for cerebral injury when TAVI is performed [6]. The well-known PARTNER trial reported an incidence of cerebrovascular stroke of 6.7% and 10% at the postoperative first month and first year following the TAVI procedure,respectively [7]. Moreover, with the advances in neuroimaging techniques and utilization of diffusion-weighted magnetic resonance imaging (DWMRI), silent cerebral ischemic events and consequent neurocognitive decline after TAVI have become evident [8]. The cerebral event may either be due to embolization during the ballooning of the aortic valve and device deployment or due to hypoperfusion during rapid ventricular pacing (RVP) and intentional hypotension [6].
The importance of a ‘heart valve team’ for the management of patients with valvular heart disease who are either candidate for SAVR or TAVI is a well-known issue in the modern management algorithm for these high-risk patients. [9]. On the other hand, the role of a proper perioperative hemodynamic management by the anaesthesiologist is essential for the overall success of the procedure in order to decrease the incidence of perioperative complications. Near infrared spectroscopy (NIRS) is a simple and cost-effective noninvasive method for evaluation of the regional oxygen saturation especially when the management of ischemic adverse cerebral and renal events is considered [10,11]. Near infrared spectroscopy and transcranial Doppler studies may provide important evidence for cerebral hypoperfusion and embolization during the perioperative period. Herein, we present our early results for the determination of cerebral ischemia using NIRS monitoring especially during rapid ventricular pacing (RVP) phase of the TAVI procedure in patients undergoing TAVI with a brief review of the literature with regard to the mechanisms of cerebrovascular complications of this procedure.
Materials and Methods
The study population included 20 consecutive patients who were scheduled for trans-femoral TAVI procedure between May 2015 and March 2016 in our institute, following the approval of the local Ethics Committee (registration and approval no: 66291034-604.01.01-E.4660). The patients were high risk candidates for conventional SAVR due to associated comorbidities (Euroscore >20). A written informed consent was signed by all the patients who were enrolled in the study. The indication for the TAVI procedure was severe aortic stenosis which was defined as aortic valve area <0.8 cm2, peak aortic jet velocity >4.0 m/sec or mean gradient >40 mmHg. The routine preprocedural evaluation included medical history, clinical examination, bilateral carotid artery Doppler ultrasonography, laboratory findings, transthoracic echocardiography, coronary, aortic and iliofemoral angiography as well as the contrast enhanced thoracic computed tomography (CT). The ventricular ejection fraction (EF), maximum and mean trans-aortic valve gradients and velocity values were measured by echocardiography and recorded before and after the procedure. Exclusion criteria were determined as severe calcification, tortuosity or a small sized iliofemoral arteries, a surgical history of aortofemoral bypass, severe aortic angulation, severe aortic arch atheroma, bleeding diathesis and recent myocardial infarction. All procedures were performed in cardiac catheterization laboratory by the same clinicians of the heart team including cardiology, anesthesiology and cardiovascular surgery.
The TAVI procedure was performed under local anesthesia with deep sedation (midazolam 0.1-0.2 mg/kg; fentanyl 2 mcg/kg and if needed propofol 0.5 mg/kg) or general anesthesia (2 MAC sevoflurane in 50% air + O2) following endotracheal intubation in 15 and 5 cases, respectively. Access site was determined based on the anatomic features (angle, tortuosity, atherosclerotic lesion etc.) of the femoral-iliac region obtained by 3-dimensionally reconstructed CT angiographic images. Once the access site was determined, 8F sheath was inserted in the common femoral artery followed by preparation for the percutaneous vascular closure system (Perclose ProGlideTM device, Abbot Vascular, IL, USA). 6F arterial sheath was inserted in the contralateral femoral artery to be used for aortic root contrast injection with a diagnostic pig-tail catheter during the procedure. Temporary RVP was obtained through the contralateral 6F femoral venous sheath. Pacing rate was adjusted at 60 bpm under VVI mode. Under fluoroscopy, the 8F arterial sheath was replaced with an 18F delivery sheath to advance the whole device into the vascular system. By use of AL1 or AL2 catheters, a hydrophilic J-tipped 0.035 inch guidewire (TerumoTM Interventional Systems, Somerset, NJ, USA) was introduced into the left ventricular cavity passing through the aortic valve and replaced with an extra-stiff 0.035 × 145 cm wire (Terumo™ Interventional Systems, Somerset, NJ, USA) to provide mechanical support for the device advancement throughout the procedure. Aortic root angiography was performed to determine the optimal fluoroscopic angle for device implantation. During aortic valve ballooning and prosthesis implantation in order to provide immobilization rapid ventricular pacing (180-200) for 10 seconds for twice was performed. The percutaneous aortic bioprosthesis system (Edwards SAPIEN XTTM, Edwards Lifesciences, Irvine, CA, USA) was implanted at the aortic level by fluoroscopic guidance under rapid pacing. Aortography was performed to check for valvular regurgitation and optimal positioning of the device with regard to the position of the coronary ostia and left ventricular outflow tract. The patient was further assessed with transesophageal echocardiography for possible complications (aortic regurgitation, dissection, pericardial effusion etc.). Once the procedure was safely terminated, the delivery system was removed and arterial puncture site was closed by use of the pre-prepared closure system. The underlying rhythm was checked and temporary pacing was continued in case of AV block or severe bradycardia. Once the hemodynamic status was considered optimal, the patient was transferred to the coronary care unit. A total of 41 TAVI procedures are performed at our institute and the abovementioned patients who were monitored with NIRS during the procedure involves the last 20 consecutive patients.
All patients were monitored with five lead-electrode ECG, a pulse oximeter, arterial blood pressure through radial route, central venous pressure, urinary catheter and temperature monitoring. Two external adhesive defibrillator pads were attached. The periprocedural cerebral oxygenation was measured with a NIRS sensor (INVOSTM-5100 C, Medtronic Inc., Minneapolis, MI, USA) located on the forehead of the patients. All of the measurements were performed at the preoperative period, at the induction and at regular time intervals (at 10., 20., 30., 45., 60. and 90. minutes). Transesophageal echocardiography was used in all cases in order to evaluate the left ventricular function, position of the implanted device and paravalvular leak at different periods throughout the procedure. Local anaesthesia and conscious sedation with intravenous midazolam (0.01-0.02 mg/kg), fentanyl (1 mcg/kg) and ketamine (1 mg/kg) were preferred in all patients. If needed, an additional 0.01 mg/kg midazolam was administered. Supplemental oxygen (FiO2: 50 %) by face mask was provided during the procedure. The setup for rapid conversion to general anaesthesia via endotracheal intubation was kept ready throughout the procedure. Lidocaine was used subcutaneously in order to achieve local anaesthesia for the insertion of radial artery and internal jugular vein catheters as well as the femoral artery puncture. Mean arterial pressure (MAP) was maintained about 65 mm Hg throughout the TAVI procedure. When a medical intervention to raise MAP was deemed necessary, ephedrine boluses were given for twice and dopamine infusion (5 mcg/kg/min) was initiated. Intravenous heparin with a dose of 70 IU/kg was administered in order to achieve an activated coagulation time of 250 seconds. All hemodynamic data and cerebral NIRS values were recorded before, during and after the procedure with constant time intervals, especially at the time of RVP and device deployment. The arterial blood gas analyses were reported at the induction, during the RVP and at the end of the procedure. The aesthetic agents and drugs used for hemodynamic stabilization as well as total dosage and duration of anaesthesia were also recorded. All of the patients were transferred to coronary intensive care unit following the procedure for observation for at least 24 hours.
The data analyses were performed using Statistical Package for the Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, IL, USA). Friedman repeated measure analysis of variance was used to compare parametric variables as well as to compare the non-parametric variables since the data were not normally distributed and the variations were not homogenous. The Wilcoxon test was used to compare the statistically significant changes in all parameters (heart rate, NIRS values etc.) between baseline and repeated measures and Bonferroni correction was made. A ‘p’ value less than 0.05 was considered as statistically significant.
Results
Twenty consecutive patients underwent the TAVI procedure via transfemoral route. The mean age was 74.4 ± 9.2 years. Male female ratio was 1.8 to 1 (13 males, 7 females). Seven patients had a left ventricular EF lower than 50 %. The mean preoperative EF was 51.7 ± 13.0. The demographic properties and operation times are presented in Table 1.
| mean | min-max | |
|---|---|---|
| Age (year) | 74.40 ± 9.28 | 50-92 |
| Height (cm) | 168.55 ± 9.61 | 150-180 |
| Weight (kg) | 81.0 ± 19.1 | 46-105 |
| Anaesthesia duration (min) | 93.75 ± 18.20 | 60-120 |
| Procedure time (min) | 70.25 ± 14.37 | 50-100 |
| Preoperative hemoglobin | 11.72 ± 1.64 | 9.3-14.4 |
| Postoperative hemoglobin | 10.2 ± 1.64 | 7.7-12.7 |
Table 1. The demographic properties and the operation times of the patients.
The mean preoperative transaortic gradient was measured to be 47.7 ± 10.4 mmHg. Mean procedure time was 70.2 ± 14.3 minutes. The rapid ventricular pacing included two episodes with a total time for pacing of 22.6 ± 5.1 seconds. Eight of the patients (40%) required ephedrine bolus during the RVP and 5 of them (25%) needed continuous dopamine infusion. The postoperative transesophageal echocardiography revealed a mean transaortic gradient of 10.1 ± 1.7 mmHg and EF of 54.11 ± 10.7% after the device deployment. The preoperative and postoperative hemodynamic status of the aortic valve and left ventricular EF are presented in Table 2. Any significant paravalvular aortic regurgitation was not encountered in any case following the device deployment.
| Preoperative | Postoperative | p | |
|---|---|---|---|
| EF (%) | 51.75 ± 13.03 | 54.11 ± 10.78 | >0.05 |
| V (m/sec) | 4.13 ± 0.92 | 1.61 ± 0.51 | 0.000* |
| Max gradient (mmHg) | 75.45 ± 15.30 | 17.07 ± 4.58 | 0.001* |
| Mean gradient (mmHg) | 47.73 ± 10.48 | 10.14 ± 1.70 | 0.001* |
EF: Ejection fraction; V: Velocity
Table 2. Preoperative and postoperative hemodynamic status of the aortic valve and left ventricular ejection fractions are presented.
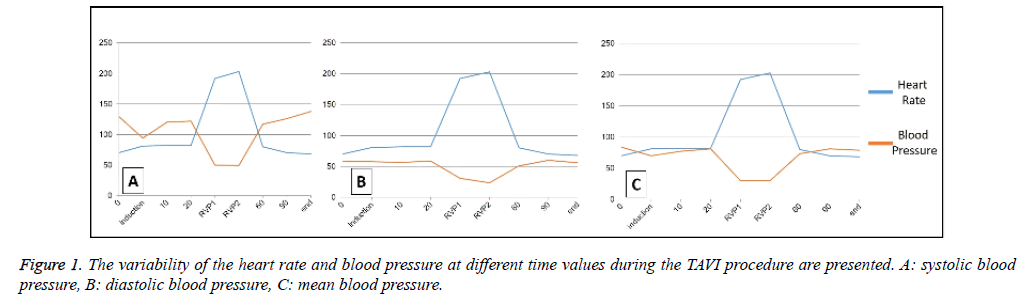
The systolic blood pressure at the preoperative period and at the induction of anaesthesia were 120 ± 30.4 mmHg and 94.6 ± 29 mmHg, respectively. The diastolic blood pressure at the preoperative period and at the induction of anaesthesia were 58.3 ± 4.7 mmHg and 58 ± 19.6 mmHg, respectively. On the other hand, the mean blood pressure at the preoperative period and at the induction of anaesthesia were 84 ± 14.5 mmHg and 70 ± 22.5 mmHg, respectively. When the heart rate was considered, at the first and the second RVP period, the heart rates were 192 ± 41 and 203 ± 32 beats/minute. The systolic/ diastolic/mean blood pressure levels at the first RVP were 50.3 ± 11.9, 31 ± 7.9 and 30.3 ± 2 mmHg, whereas the same values for the second RVP were 49.6 ± 13.3, 24.6 ± 5 and 30 ± 9.8 mmHg. The variability of the heart rate and blood pressure at different time values during the TAVI procedure are presented in Figure 1.
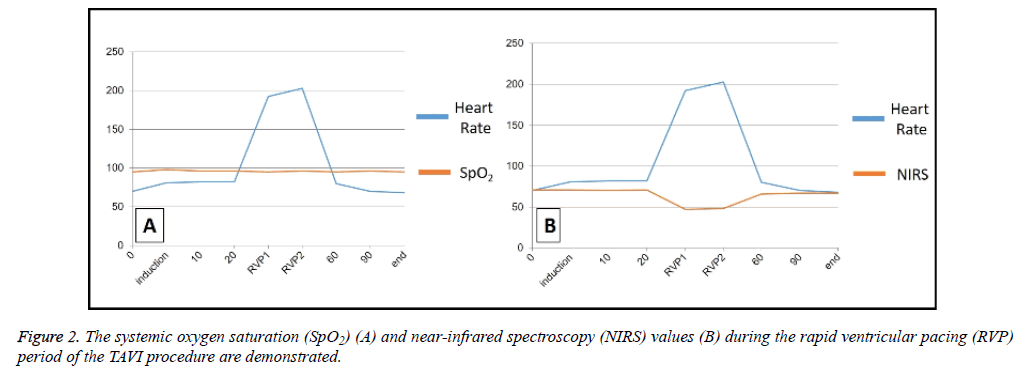
There was a statistically significant difference with regard to the heart rate and the cerebral NIRS values (p=0.006 and p=0.02; respectively) in all patients during the RVP period. The cerebral NIRS values were statistically lower than baseline (pre-RVP) levels (p<0.001). Figure 2 represents the variability in systemic oxygen saturation and RVP period during the procedure.
Any intraoperative mortality was not encountered in the study group. One patent was lost due to a sudden cardiac death at home 15 days after the TAVI procedure. Any cerebrovascular event was not encountered in the study population.
Discussion
Determination of the factors that influence the clinical results and patient survival is of paramount importance in terms of both patient selection and precautions to increase the complications. The recent indications for TAVI are as follows: (i) patients who meet an indication for SAVR but having a prohibitive risk for SAVR and a predicted post-TAVI survival greater than 12 months (class I, level of evidence: B); (ii) TAVI is a reasonable alternative to SAVR in patients who meet an indication for SAVR and who have high surgical risk (class IIa, level of evidence: B) [9]. Valve Academic Research Consortium (VARC) defines the possible complications after TAVI which may be encountered up to one third of the patients during the first month as death, cerebrovascular events, vascular events, bleeding, acute kidney injury, paravalvular regurgitation, valve malpositioning, coronary obstruction, myocardial infarction and cardiac conduction abnormalities [12,13].
Stroke is an important periprocedural complication of TAVI and its fundamental definition is even endorsed by Food and Drug Administration (FDA) in order to further evaluate the consequences of cerebral injury during the TAVI procedure [13]. An entity closely related to clinical stroke is the transient ischemic attack (TIA), which in fact presents a transient episode of focal neurological dysfunction without acute infarction [13]. The etiology of the cerebral ischemic events may be either embolization or a state of hypoperfusion. The clinical consequence of the stroke may further be classified as disabling or non-disabling. Embolic showering of the cerebral circulation may occur at any stage of the TAVI procedure, potentially leading to periprocedural acute stroke [14]. The most important monitoring methods for embolization and hypoperfusion are transcranial Doppler ultrasonography and NIRS, respectively. Several studies have emphasized the association between the number of high-intensity transient signals and cerebral embolization, which may be encountered at any procedural phase of the TAVI procedure [15-17]. Balloon aortic valvuloplasty is an important step during TAVI to facilitate the delivery of the undeployed valve. The rapid ventricular pacing and BAV period of the TAVI procedure results in an intentional state of systemic hypotension which may lead to cerebral hypoperfusion. Elevated heart rate could negatively influence cardiovascular risk in the general population. Furthermore, elevated heart rate can directly increase heart ischemic conditions because of its skill in unbalancing demand/supply of oxygen and decreasing the diastolic period [18]. These episodes of systemic hypotension may induce cerebral ischemia in watershed territories, as well as impairing the washout of dislodged microemboli. Moreover, this period of cerebral hypoperfusion may accentuate the microembolic ischemic effects due to reduced effectiveness of the circulation to washout debris promptly [14].
Mixed venous oxygen saturation is still the gold standard for the determination of the systemic oxygen delivery to consumption in patients undergoing cardiac surgery [19]. Although the exact determination of mixed venous oxygen saturation may be evaluated with jugular bulb venous oxygen saturation, NIRS may provide with an easier and noninvasive method for determination of the regional cerebral oxygen saturation (rScO2). The relation between the NIRS parameters and systemic or cerebral perfusion determination during the RVP and device deployment phase of TAVI procedures is very limited in the literature [18,19]. Our study revealed close relationship between the cerebral NIRS values during systemic hypotension which is created intentionally during the RVP phase of the TAVI procedure. Although we did not encounter any cerebrovascular complication during the perioperative period, the risk of cerebral ischemia may increase during the RVP when the NIRS values remain significantly low. The respiratory management of these patients is not always effective when the procedure is performed with sedation and spontaneous respiration. The elevated carbondioxide levels leading to increased partial carbondioxide tension and a consequent increase in cerebral perfusion is speculated to be a preventive measure [20]. However, respiratory depression, hypercarbia and acidosis may lead to increased pulmonary artery pressure which may lead to right ventricular failure [21]. This untoward effect may be significant since up to 50% of the patients undergoing TAVI procedure are known to have pulmonary hypertension [22]. In our limited experience, we allowed the partial carbon dioxide pressure to riseup to 50 mmHg in intubated patients with decreasing the tidal volume and respiration frequency. Nevertheless, the carbon dioxide levels remained in low to normal levels in patients whom the procedure was performed under sedation and spontaneous respiration. However, we did not encounter a statistically significant difference between these two management algorithms.
Limitations of the Study
The most important limitation of the study is the small number of the patient population. Because of the rare need for TAVI performance, small sample size may be acceptable. Secondly, for the time being, we do not have any routine implementation of transcranial Doppler evaluation during the TAVI procedure in our catheterization laboratory. Further studies in order to correlate NIRS, transcranial Doppler ultrasonography and electroencephalography monitoring are deemed necessary in order to prevent the cerebrovascular complications of the TAVI procedure.
Conclusion
This observational study presents the significant decrease of cerebral NIRS values during the rapid ventricular pacing phase of the TAVI procedure. Although we do not have any data about the additive effect of this method, we may speculate that further studies may reveal cut-off values both for NIRS values and rapid ventricular pacing duration in order to set a critical level for increased cerebrovascular complication risk during the device deployment in TAVI procedure.
References
- Aksu T, Yüksel UC, Tuzcu M. Percutaneous treatment of aortic stenosis. Turk Kardiyol Dern Ars 2010; 38: 290-301.
- Roques F, Nashef SA, Michel P; EuroSCORE study group. Risk factors for early mortality after valve surgery in Europe in the 1990s: lessons from the EuroSCORE pilot program. J Heart Valve Dis 2001; 10: 572-577.
- Cribier A, Savin T, Berland J, Rocha P, Mechmeche R. Percutaneous transluminal balloon valvuloplasty of adult aortic stenosis: report of 92 cases. J Am Coll Cardiol 1987; 9: 381-386.
- Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Sebagh L. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43: 698-703.
- Tuzcu EM. Editorial Comment: going behind the ordinary: transcatheter aortic valve implantation. Turk Kardiyol Dern Ars 2010; 38: 264-266.
- Hauville C, Ben-Dor I, Lindsay J, Pichard AD, Waksman R. Clinical and silent stroke following aortic valve surgery and transcatheter aortic valve implantation. Cardiovasc Revasc Med 2012; 13: 133-140.
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363: 1597-1607.
- Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 2010; 121: 870-878.
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129: e521-643.
- Örmeci T, Alkan-Bozkaya T, Özyüksel A, Ersoy C, Ündar A, Akçevin A. Correlation between cerebral-renal near-infrared spectroscopy and ipsilateral renal perfusion parameters as clinical outcome predictors after open heart surgery in neonates and infants. Artif Organs 2015; 39: 53-58.
- Ersoy C, Özyüksel A, Alkan Bozkaya T, Karaaslan P, Örmeci T, Undar A. Are perioperative near-infrared spectroscopy values correlated with clinical and biochemical parameters in cyanotic and acyanotic infants following corrective cardiac surgery? Perfusion 2016; 31: 125-130.
- Malaisrie SC, Iddriss A,, Flaherty JD, Churyla A. Transcatheter Aortic Valve Implantation. Curr Atheroscler Rep 2016; 18: 27.
- Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg 2013; 145: 6-23.
- Hynes BG, Rodés-Cabau J. Transcatheter aortic valve implantation and cerebrovascular events: the current state of the art. Ann NY Acad Sci 2012; 1254: 151-163.
- Szeto WY, Augoustides JG, Desai ND, Moeller P, McGarvey ML, Walsh E. Cerebral embolic exposure during transfemoral and transapical transcatheter aortic valve replacement. J Card Surg 2011; 26: 348-354.
- Kahlert P, Al-Rashid F, Döttger P, Mori K, Plicht B, Wendt D.. Cerebral embolization during transcatheter aortic valve implantation: a transcranial Doppler study. Circulation 2012; 126: 1245-1255.
- Drews T, Pasic M, Buz S, Unbehaun A, Dreysse S, Kukucka M. Transcranial Doppler sound detection of cerebral microembolism during transapical aortic valve implantation. Thorac Cardiovasc Surg 2011; 59: 237-242.
- Scicchitano P, Cortese F, Ricci G, Carbonara S, Moncelli M. Ivabradine, coronary artery disease, and heart failure: beyond rhythm control. Drug Des Devel Ther 2014; 8: 689-700.
- Paarmann H, Heringlake M, Heinze H, Hanke T, Sier H, Karsten J. Non-invasive cerebral oxygenation reflects mixed venous oxygen saturation during the varying haemodynamic conditions in patients undergoing transapical transcatheter aortic valve implantation. Interact Cardiovasc Thorac Surg 2012; 14: 268-272.
- Mayr NP, Hapfelmeier A, Martin K, Kurz A, van der Starre P, Babik B. Comparison of sedation and general anaesthesia for transcatheter aortic valve implantation on cerebral oxygen saturation and neurocognitive outcome. Br J Anaesth 2016; 116: 90-99.
- Mazzitelli D, Lange R, Wiesner G, Tassani-Prell P. Perioperative risk and management in patients with pulmonary hypertension. Minai OA, Yared JP, Kaw R, Subramaniam K, Hill NS. Chest 2013; 144: 329-340.
- Ben-Dor I, Goldstein SA, Pichard AD, Satler LF, Maluenda G, Li Y. Clinical profile, prognostic implication, and response to treatment of pulmonary hypertension in patients with severe aortic stenosis. Am J Cardiol 2011; 107: 1046-1051.