ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 13
Drug utilization evaluation of cephaosporins in a tertiary care hospital: inpatient departments
Mohd. Mahmood*, Ramya Mounika A, Pranali S. Pandit, Niveditha B and Rahul Kumar P
Malla Reddy Pharmacy College, Hyderabad, Telangana, India
- *Corresponding Author:
- Mohd. Mahmood
Malla Reddy Pharmacy College
Hyderabad, India
E-mail: drmahmood7788@gmail.com
Accepted on June 19, 2017
Background: As bacterial resistance has grown due to inappropriate use of antibiotics we sought to evaluate the current utilization of cephalosporins in inpatient departments, in terms of rational usage towards indication, dosage, dosing regimen, and dosage adjustments in comorbid conditions (renal and hepatic impairments). Drug utilization evaluation is an ongoing, authorized, quality improvement process which helps in evaluation and improvement of drug usage, by analyzing the medication prescribing, administration, or dispensing process. By developing criteria and standards which help in appropriate drug use and educating health care professionals in accordance with rational use of drugs to achieve optimal patient outcomes.
Methods: The study design was, prospective and concurrent Drug Utilization Evaluation. This study was carried out for a period of 6 months between October 2016 to March 2017. This study is divided into phase I (before intervention) and phase II (after intervention).This study includes all the patients who have been prescribed with any generation of cephalosporins. The criteria and guidelines were taken from the standard references and used to monitor the drug therapy on patients based on indication, dose, dosage form, frequency, duration, laboratory tests. All the standard guidelines for prescribed cephalosporins have been circulated to the required physicians and other related health care professionals.
Results: Inappropriateness of drug therapy was found in phase I (before intervention) and appropriateness in terms of rational use for indication, dose, dosing interval, renal dosage adjustment was improved in phase II this may be due to the implementation of the DUE guidelines through interventions obtained during phase.
Keywords
Drug utilization evaluation, Cephalosporins, DDD, DID, PDD, Indication, Interventions, Appropriateness, Inappropriateness, Rational use, Brand name, Generic name, Culture, Sensitivity testing.
Introduction
Definition
According to WHO, Drug Utilization evaluation is defined as the marketing, distribution, prescription and use of drugs in society, with special emphasis on the resulting medical, social and economic consequences [1]. Drug Utilization Evaluation (DUE) is an ongoing authorized and systematic quality improvement process, designed to
• To optimize drug use by developing criteria and standards.
• To educate clinicians and other Health Care Professionals (HCP), to increase appropriate drug use.
• To provide feedback of results obtained during study to clinicians and other HCP.
• To review drug use.
• To analyse prescription pattern [2].
Types of DUE
Drug focused: Drug utilization evaluation of a single drug (e.g. Ceftriaxone) or a class of drugs (e.g. Cephalosporins) is tested.
Indication focused: Evaluation of drug or drugs that is used for specific indication is examined for their use.
Quantitative: It includes collecting, organizing and estimation of drug usage in figures in the pattern of drug acquisition, prescribing, dispensing, consumption and distribution.
Qualitative: This type of DUE helps in evaluating the quality of drug therapy and its outcomes by comparing practice with predetermined criteria and standards [2,3].
Role of pharmacist in DUE
• Performing pilot studies, collection of data, analyzing collected data and writing a report.
• To plan, organize and implement a DUE program.
• Developing, supervising and coordination of DUE program.
• To promote goals and objectives of DUE.
• To document outcomes of program its effectiveness and cost benefits.
• To present DUE results that obtained at meetings and conferences.
• To educate hospital about DUE and its use [5].
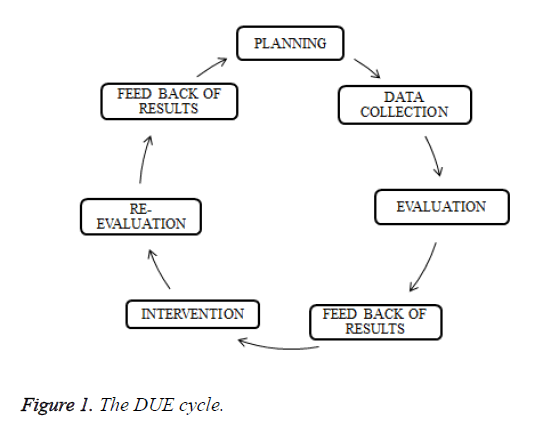
The DUE cycle
The DUE cycle is shown in Figure 1.
Metrics used in drug utilization evaluation
Defined daily dose (DDD): The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults. It can be done to only products which is approved and marketed at least in one country.
DDDs/1000 inhabitants/day: It provides a rough estimate of the quantity of drug that is used in a proportion of specific population. This kind of DDD is mostly used for estimating drugs that are used chronically.
DDDs/1000 people/d=(amount drug (mg) × 1000)/(DDD × 365 d × sample size)
Prescribed daily dose/consumed daily dose: It is defined as an average dose prescribed according to a representative sample of prescriptions. This gives the information about average amount of a drug that is actually prescribed on daily basis [1].
BMI: It is defined as the weight in kilograms divided by square of height in meters. It is a ratio of weight to height usually used to classify individuals with overweight, underweight and obesity.
Creatinine clearance: Creatinine clearance is the amount of plasma or blood that is cleared of creatinine this used as a tool to measure the kidney functioning. This can be calculated using Cockcroft-Gault equation.
If female, Ccr=(((140-age) × weight)/(72 × Scr)) × 0.85
If male, Ccr=(((140-age) × weight)/(72 × Scr)) × 1 [5]
Cephalosporins
Cephalosporins are first obtained from isolated cultures of Cephalosporium acremonium. Cephalosporins acts as bactericidal compounds, with the help of β-lactum ring structure by interfering with the synthesis of bacterial cell wall [6].
Cephalosporins are classified by generation (Table 1). Each individual cephalosporins differ from their action by showing difference in,
• Susceptibility towards microorganism
• Pharmacokinetic properties
• Binding capability towards receptor (PBP s) [7]
| First generation cephalosporins | |
|---|---|
| Parenteral | Oral |
| Cefazolin | Cephalexin |
| Cefadroxil | |
| Second generation cephalosporins | |
| Parenteral | Oral |
| Cefuroxime | Cefaclor |
| Cefoxitin | Cefuroxime axetil |
| Cefprozil | |
| Third generation cephalosporins | |
| Parenteral | Oral |
| Cefotaxime | Cefixime |
| Ceftizoxime | Cefpodoximeproxetil |
| Ceftriaxone | Cefdinir |
| Ceftazidime | Ceftibuten |
| Cefoperazone | Ceftametpivoxil |
| Fourth generation cephalosporins | |
| Parenteral | |
| Cefepime | |
| Cefpirome | |
Table 1. Different generations of cephalosporins.
Third generation cephalosporins are less active against gram positive bacteria but they are highly effective against gram negative rods. These are most commonly used for hospital acquired infection such as bacterial pneumonia [8].
Among four generations of cephalosporins third generation cephalosporins like cefotaxime, ceftriaxone are most commonly used. Resistant to these drugs might be due to production of extended spectrum β lactases by organisms [9].
Aim and Objectives
Aim
To study the drug utilization and evaluation of cephalosporins in the inpatient wards of a tertiary care hospital.
Objectives
• To decrease the medication error and their related problems
• To observe the prescribing pattern of cephalosporins
• To assess the rationality of prescribing
• To check for cost effectiveness of therapy
• To rationalize drug use by providing criteria and standards
Methodology
Study site
Mediciti institute of medical sciences
Study design
Prospective study
Study duration
Six months from October 2016 to March 2017.
Study details
DUE study was carried out in patients prescribed with cephalosporins. The study period was 6 months divided into 2 phases. Phase 1 from October 2016 to December 2016 and phase 2 for a period of 3 months from January 2017 to March 2017.
Plan of work
Phase 1
Step 1: Approval from head of the department and hospital authorities.
Step 2: Literature review.
Step 3: Designing data collection form.
Step 4: Identification of patients with cephalosporin therapy
and recording the data.
Step 5: Evaluating the recorded data.
Step 6: Preparation and circulation of standard guidelines with respect to obtained data.
Phase 2
Step 7: Identification of patients with cephalosporin therapy and recording the data.
Step 8: Evaluating the collected data.
Step 9: Feedback of results to physicians and other HCP
Results and Discussion
Results
In this study, we have enrolled 202 patients who have been prescribed with cephalosporins. The appropriateness for indication, dose, dosing interval, dosage adjustment in renal impairment patients, were assessed.
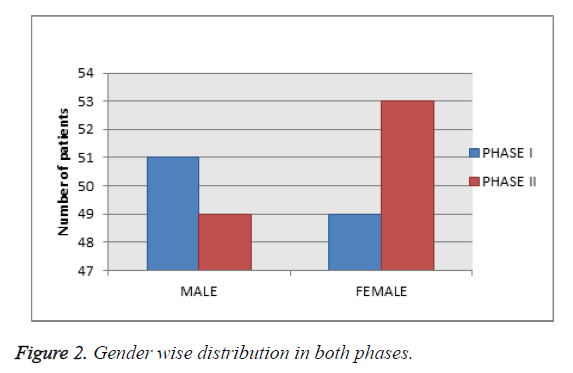
Gender wise distribution in both phases
Among 202 patients enrolled in the study, as shown in Table 2 and Figure 2, 52% were male and 48% were female.
| S. no | Gender | No of patients in phase I | No of patients in phase II |
|---|---|---|---|
| 1 | Males | 51 | 49 |
| 2 | Females | 49 | 53 |
Table 2. Gender wise distribution in both phases.
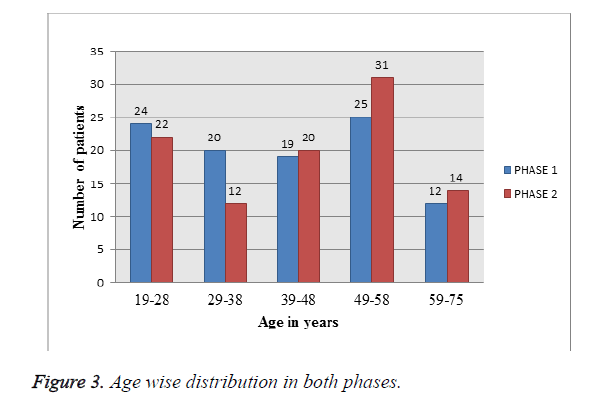
Age wise distribution in both phases: Among 202 patients enrolled in the study, as depicted in Figure 3 and Table 3, Patients in between age group of 49-58 y are most predominantly prescribed with cephalosporins in both phases.
| s. no | Age | No. of patients in Phase I | No. of patients in Phase II |
|---|---|---|---|
| 1 | 19-28 y | 24 | 22 |
| 2 | 29-38 y | 20 | 12 |
| 3 | 39-48 y | 19 | 20 |
| 4 | 49-58 y | 25 | 34 |
| 5 | 59-75 y | 12 | 14 |
Table 3. Age wise distribution in both phases.
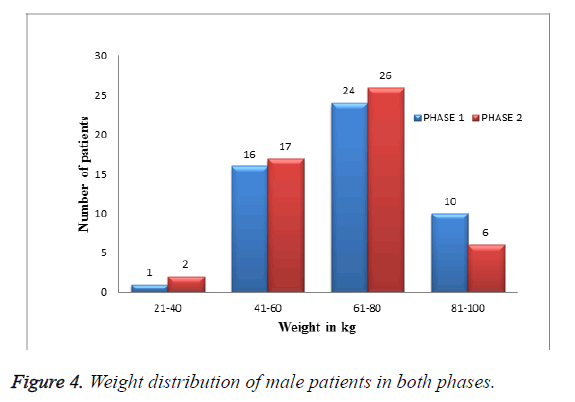
Weight distribution of male patients in both phases: Among 202 patients enrolled in the study, 96 were male patients. As shown in Table 4 and Figure 4 In them more patients were less than 61-80 kg of weight distribution.
| S. no | Weight | No. of patients in Phase I | No. of patients in Phase II |
|---|---|---|---|
| 1 | 21- 40 kg | 1 | 2 |
| 2 | 41-60 kg | 16 | 17 |
| 3 | 61-80 kg | 24 | 26 |
| 4 | 81-100 kg | 10 | 6 |
Table 4. Weight distribution of male patients in both phases.
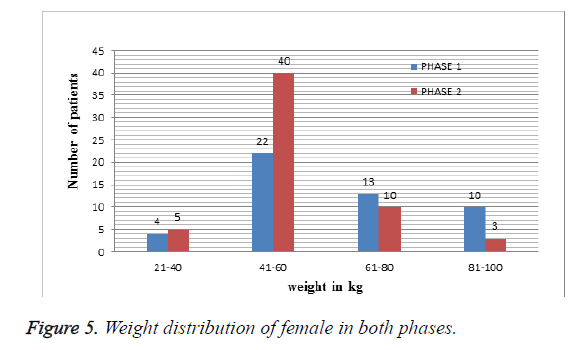
Weight distribution of female patients in both phases: Among 202 patients enrolled in the study, 106 were female patients. As shown in Table 5 and Figure 5 more patients were less than 41-60 kg of weight distribution.
| S. no | Weight | No. of patients in Phase I | No. of patients in Phase II |
|---|---|---|---|
| 1 | 21- 40 kg | 4 | 5 |
| 2 | 41-60 kg | 22 | 39 |
| 3 | 61-80 kg | 13 | 10 |
| 4 | 81-100 kg | 10 | 3 |
Table 5. Weight distribution of female patients in both phases.
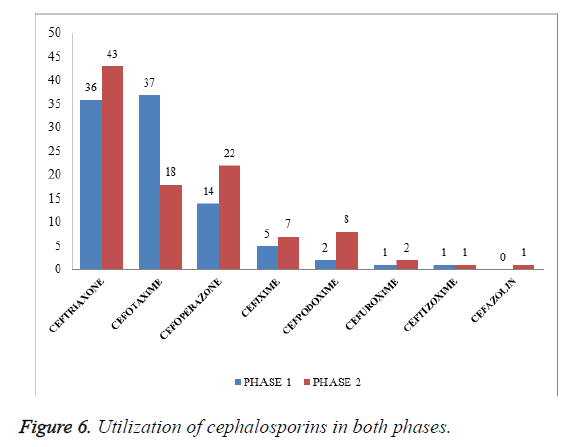
Utilization of cephalosporin in both phases: Among 202 patients enrolled in the study, as shown in Table 6 and Figure 6, 79 patients were prescribed with ceftriaxone followed by cefotaxime 55 (37 in phase I, 18 in phase II), cefoperazone 36 (14 in phase I, 22 in phase II), cefixime 12 (5 in phase I, 7 in phase II), cefpodoxime 10 (2 in phase I, 8 in phase II), cefuroxime 3 (1 in phase I, 2 in phase II), ceftizoxime 2 (1 in phase I, 1 in phase II), cefazolin was given for 1 patient in phase II. Cefotaxime was prescribed predominantly in phase I in contrast with phase II ceftriaxone was predominantly prescribed after intervention may be due to its high broad spectrum of activity.
| Drug | No. of patients in phase I | No of patients in phase II |
|---|---|---|
| Ceftriaxone | 36 | 43 |
| Cefotaxime | 37 | 18 |
| Cefoperazone | 14 | 22 |
| Cefixime | 5 | 7 |
| Cefpodoxime | 2 | 8 |
| Cefuroxime | 1 | 2 |
| Ceftizoxime | 1 | 1 |
| Cefazolin | 1 |
Table 6. Utilization of cephalosporins in both phases.
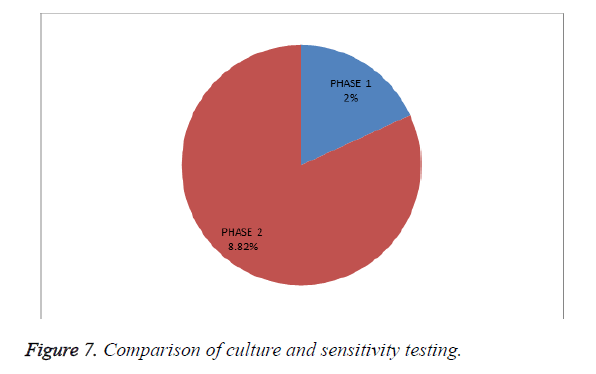
Culture and sensitivity testing (outcome indicator): Among 202 patients enrolled in the study, as shown in Table 7 and Figure 7, culture and sensitivity testing was performed for 2 patients in phase I and after intervention it has improved. This test should be considered before prescribing cephalosporin to provide appropriate and rational therapy.
| S. no | Phases | Total no. of patients | Culture and sensitivity performed |
|---|---|---|---|
| 1 | Phase I | 100 | 2 (2%) |
| 2 | Phase II | 102 | 9 (8.82%) |
Table 7. Comparison of culture and sensitivity testing.
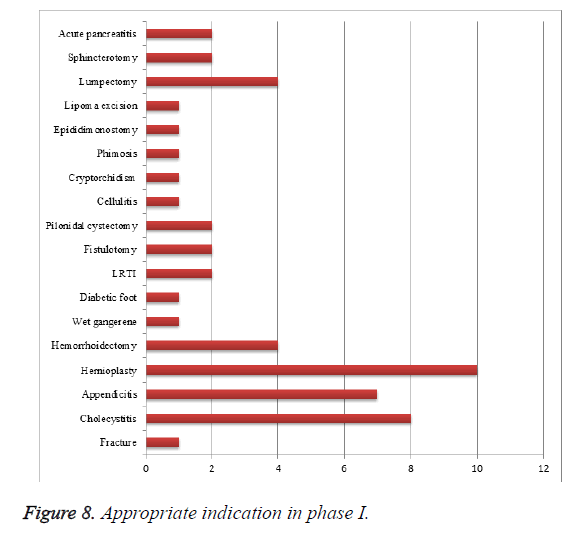
Rational drug therapy of cephalosporins in phase I for the following indication: Among 100 patients enrolled in the study, 51 patients were prescribed rationally for their condition (Table 8 and Figure 8). As of our study irrational use of cephalosporins was found to be high, this may be improved by considering antibiotics criteria and standards while prescribing cephalosporins.
| S. no | Indication | No. of patients |
|---|---|---|
| 1 | Fracture | 1 |
| 2 | Cholecystitis | 8 |
| 3 | Appendicitis | 7 |
| 4 | Hernioplasty | 10 |
| 5 | Hemorrhoidectomy | 4 |
| 6 | Wet gangerene | 1 |
| 7 | Diabetic foot | 1 |
| 8 | LRTI | 2 |
| 9 | Fistulotomy | 2 |
| 10 | Pilonidal cystectomy | 2 |
| 11 | Cellulitis | 1 |
| 12 | Cryptorchidism | 1 |
| 13 | Phimosis | 1 |
| 14 | Epididimonostomy | 1 |
| 15 | Lipoma excision | 1 |
| 16 | Lumpectomy | 4 |
| 17 | Sphincterotomy | 2 |
| 18 | Acute pancreatitis | 2 |
Table 8. Appropriate therapy for indication phase I.
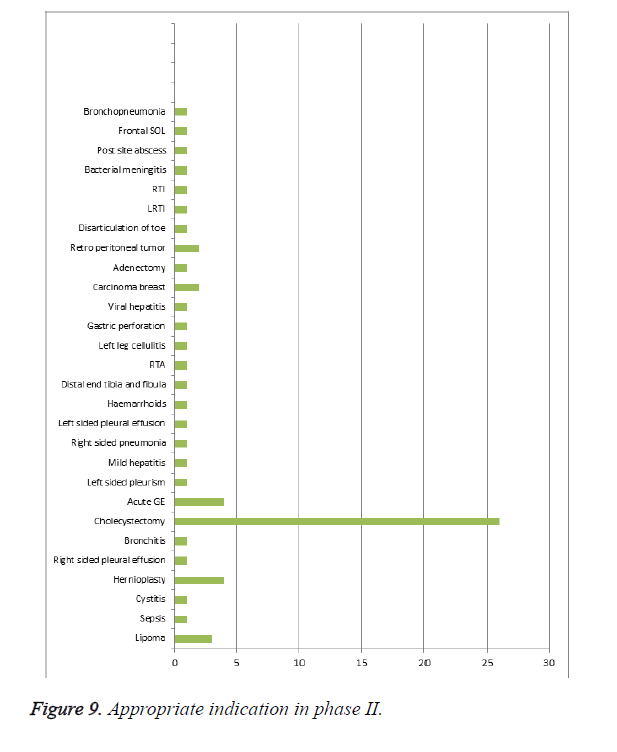
Rational drug therapy of cephalosporins in phase II for the following condition: Among 102 patients enrolled in the study, as shown in Table 9 and Figure 9, 64 patients were prescribed rationally for their condition. When compared to phase I appropriate therapy for indication was increased in phase II.
| S. no | Indication | No. of patients |
|---|---|---|
| 1 | Lipoma | 3 |
| 2 | Sepsis | 1 |
| 3 | Cystitis | 1 |
| 4 | Hernioplasty | 4 |
| 6 | Right sided pleural effusion | 1 |
| 7 | Bronchitis | 1 |
| 8 | Cholecystectomy | 26 |
| 9 | Acute GE | 4 |
| 10 | Left sided pleurism | 1 |
| 12 | Mild hepatitis | 1 |
| 13 | Right sided pneumonia | 1 |
| 14 | Left sided pleural effusion | 1 |
| 16 | Haemarrhoids | 1 |
| 17 | Distal end tibia and fibula | 1 |
| 18 | RTA | 1 |
| 19 | Left leg cellulitis | 1 |
| 20 | Gastric perforation | 1 |
| 21 | Viral hepatitis | 1 |
| 22 | Carcinoma breast | 2 |
| 23 | Adenectomy | 1 |
| 24 | Retro peritoneal tumor | 2 |
| 25 | Disarticulation of toe | 1 |
| 26 | LRTI | 1 |
| 27 | RTI | 1 |
| 28 | Bacterial meningitis | 1 |
| 29 | Post site abscess | 1 |
| 30 | Frontal SOL | 1 |
| 31 | Bronchopneumonia | 1 |
Table 9. Appropriate therapy for indication phase II.
ATC/DDD classification with calculated DID values for cephalosporins in phase-I: As shown in Table 10, DID for ceftriaxone can be interpreted as 4.44 out of 1000 patients or 44.4% patients would have used a dose of 2 g, the DID of cefotaxime, cefoperazone, cefuroxime, cefixime, ceftizoxime, cefpodoxime can be interpreted as the consumption of their respective DDDs by population of 41.1%, 15.5%, 1.1%, 0.5%, 1.1%, 0.2%, patients. The ratio of PDD/DDD is often used to check the adequacy of dosing. A ratio of <1 was seen in cefotaxime and ceftizoxime indicates under dosing, a ratio of >1 was seen in ceftriaxone indicates over dosing. All the other cephalosporins showed a PDD/DDD ratio equal to 1 indicates adequacy of dosing in these cases.
| Drug | ATC/DDD code | DDD (g) | DDD/1000 inhabitant/d | PDD (g) | PDD/DDD |
|---|---|---|---|---|---|
| Ceftriaxone | J01DD04 | 80 | 4.44 | 84 | 1.05 |
| Cefotaxime | J01DD01 | 148 | 4.11 | 74 | 0.5 |
| Cefoperazone | J01DD12 | 42 | 1.55 | 42 | 1 |
| Cefuroxime | J01DC02 | 3 | 0.11 | 3 | 1 |
| Cefixime | J01DD08 | 2 | 0.05 | 2 | 1 |
| Ceftizoxime | J01DD07 | 4 | 0.11 | 2 | 0.5 |
| Cefpodoxime | J01DD13 | 0.8 | 0.02 | 0.8 | 1 |
Table 10. Defined daily dose for phase I.
ATC/DDD classification with calculated DID values for cephalosporins in phase II: As shown in Table 11, DID for ceftriaxone can be interpreted as 4.68 out of 1000 patients or 46.8% patients would have used a dose of 2 g similarly, the DID of cefotaxime, cefoperazone, cefuroxime, cefixime, ceftizoxime, cefpodoxime can be interpreted as the consumption of their respective DDDs by population of 19.6%, 23.9%, 2.1%, 7.6%, 1%, 1%, patients. A ratio of <1 was seen in cefotaxime and indicates under dosing, all the other cephalosporins showed a PDD/DDD ratio equal to 1 indicates adequacy of dosing in these cases. In phase I three drugs were prescribed inadequately but in phase II, inadequacy was seen only in one drug.
| Drug | ATC/DDD Code | DDD (g) | DDD/1000 inhabitant/d | PDD (g) | PDD/DDD |
|---|---|---|---|---|---|
| Ceftriaxone | J01DD04 | 86 | 4.68 | 86 | 1 |
| Cefotaxime | J01DD01 | 72 | 1.96 | 52 | 0.72 |
| Cefoperazone | J01DD12 | 66 | 2.39 | 66 | 1 |
| Cefuroxime | J01DC02 | 6 | 0.21 | 6 | 1 |
| Cefixime | J01DD08 | 2.8 | 0.76 | 2.8 | 1 |
| Ceftizoxime | J01DD07 | 4 | 0.10 | 4 | 1 |
| Cefazolin | J01DD04 | 3 | 0.10 | 3 | 1 |
| Cefpodoxime | J01DD13 | 3.2 | 0.87 | 3.2 |
Table 11. Defined daily dose for phase II.
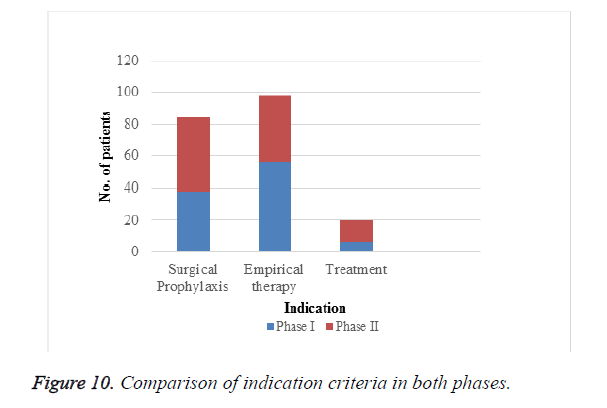
Indication criteria: Among 202 patients enrolled in the study as depicted in Figure 10 and Table 12, empirical therapy was more in phase II than in phase I and prescribing for actual treatment was also improved in phase II that is after intervention. According to our study we found that appropriate use of cephalosporins for actual treatment was low which should be encouraged.
| Criteria | Phase I | Phase II |
|---|---|---|
| Surgical prophylaxis | 38 | 46 |
| Empirical therapy | 56 | 42 |
| Treatment | 6 | 14 |
Table 12. Indication criteria.
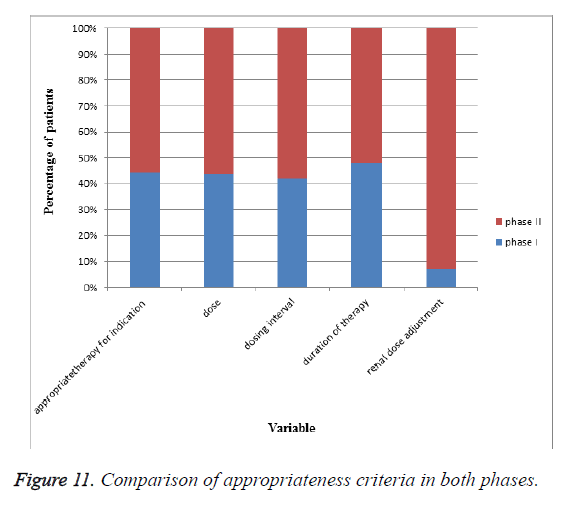
Appropriateness of antibiotic therapy in both phases: In our study we enrolled 202 patients prescribed with cephalosporins. As shown in Table 13 and Figure 11 appropriateness in terms of indication for treatment, dose, duration of therapy, dosing interval, renal dosage adjustment were found to be low and to encourage rational use, we obtained and circulated criteria and standards to required health care professionals. After intervention appropriateness in terms of indication for treatment, dose duration of therapy, dosing interval, and renal dosage adjustment was improved.
| S. no | Variable | Appropriateness in phase I | Appropriateness in phase II2 (%) |
|---|---|---|---|
| 1 | Indication for treatment | 51 (51%) | 64 (62.74%) |
| 2 | Dose | 52 (52%) | 67 (65.68%) |
| 3 | Duration of therapy | 62 (62%) | 86 (84.31%) |
| 4 | Dosing interval | 75 (75%) | 82 (80.39%) |
| 5 | Dose adjustment for crcl<50 ml/min | 1 (1%) | 11.50 (13) |
Table 13. Appropriateness of antibiotic therapy in phase I and phase II.
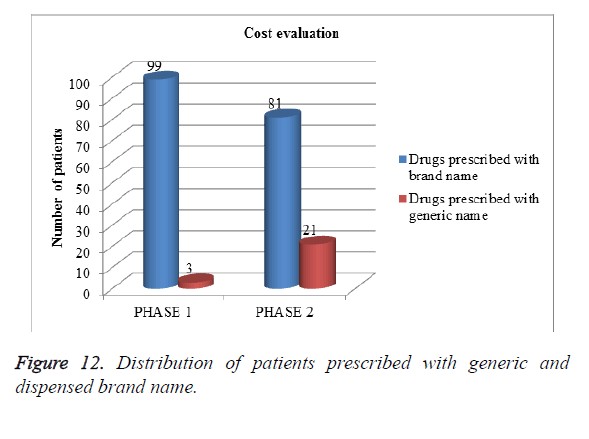
Evaluation of cost in both phases: Among 202 patients enrolled in the study, as shown in Figure 12, more patients were prescribed with dispensing brand name rather than generic name but after intervention prescribing with generic name was slightly improved. Generic drugs are cheaper in cost when compared to brand drugs (Table 14). In our study cost evaluation was done by comparing number patients prescribed with generic name and dispensing brand name.
| Phases | Drugs prescribed with brand name | Drugs prescribed with generic name |
|---|---|---|
| Phase I | 99 | 3 |
| Phase II | 81 | 21 |
Table 14. Cost evaluation.
Discussion
Phase I (before intervention)
A total of 100 patients were enrolled in phase I, among them irrational therapy for indication was found in 51% of patients and rational therapy was found in 49% of patients, rational dose was given to 52% and inappropriate dose was administered to 48% of patients, duration of therapy was appropriate for 62% and inappropriate for 48%of patient. Appropriateness in terms of indication for treatment, dose, duration of therapy, dosing interval was found to be low this may be improved by considering antibiotic guidelines before prescribing.
Dose adjustments for patients with GFR<50 ml/min was performed for 2 patients among 14 patients. Culture and sensitivity testing which gives information about bacterial eradication and sensitivity towards can be justified by obtaining susceptible organism before starting the therapy with cephalosporins and negative culture within 24 h after discontinuation of therapy. This was performed only for 2 patients during phase I.
Phase II (after intervention)
Phase II study carried out after providing criteria and standards to health care professional. A total of 102 patients were enrolled, among them there is slight improvement in rational therapy for indication i.e. from 52% to 62.74%.
Rational dose administration was increased than in phase I i.e., from 52 to 67%.
Dose adjustments for patients with creatinine clearance rate<50 ml/min was performed in 13 patients among 19 patients renal dosage adjustment was found to be high than in phase I.
Culture and sensitivity testing was performed in 9 patients among 102 patients prescribed with cephalosporins. There is very little improvement in considering culture and sensitivity test before and after administration of cephalosporins than in phase I. culture and sensitivity test should be given importance before considering the therapy, and after discontinuing the therapy to see the effectiveness of the drug.
Conclusion
Rational use of cephalosporins in accordance with appropriate drug for indication, appropriate dose, dosing interval, duration of therapy, renal dosage adjustment for specific indication and particular individual was found to be low in phase I and rational use was improved after intervention in phase II. By implementing criteria and standards rational drug therapy can be achieved. Antibiotic guidelines, criteria and standard should be considered before prescribing antibiotics. Prescribing pattern in terms of generic name was found to be low this should be encouraged to provide cost effective treatment to the patient as generic drugs are cheaper in cost when compared to brand ones. Physicians should order culture and sensitivity testing despite of patients’ financial burden, to provide rational drug therapy and to prevent bacterial resistance. Rational use of antibiotics should be encouraged.
Future Perspectives
By implementing criteria and standards in hospitals rational use of drugs can be improved.
Further DUE studies can be conducted on drugs with more ADR profiles, drugs with narrow therapeutic index and drugs that are most widely used.
This type of DUE studies in other class of antibiotics, may improve prescribing pattern in terms of rational therapy and also to prevent resistance towards bacteria.
References
- Essential Medicines and Health Products Information Portal A World Health Organization resource: introduction to drug utilization research, 2003.
- Parthasarathi G, Karin NH, Milap C. A text book of clinical pharmacy practice. Essential Concepts Skills 2005; 364-371.
- Kiran N. Drug utilization evaluation of cephalosporins, macrolides, quinolones antibiotics in KIMS Hospital. Int J Res Pharm Chem 2014; 4: 841-849.
- Lakshmi S. Drug utilization evaluation for post-operative patients in obstetrics and gynaecology department in a tertiary care teaching hospital. World J Pharm Pharmac Sci 2016; 5: 1342-1356.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31-41.
- Bushra R. Evaluation of the use of cephalosporin antibiotics in pediatrics. J Appl Pharm Sci 2013; 3: 63-66.
- Tripathi KD. Essentials of Medical Pharmacology (7th Edn.) 2013; 725-730.
- Jawetz MA. Medical microbiology. McGraw Hill New York (23rd Edn.) 2004.
- Rekha B. Utilization of third generation cephalosporins in multispeciality teaching hospital. Ind J Pharm Pract 2009; 2.