ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2007) Volume 18, Issue 2
Distribution of major serum proteins in an airbrea-thing teleost, Channa punctata Bl. (Channidae: Channiformes)
1Section of Genetics, Department of Zoology, Faculty of Life Science; A. K. Tibbiya College; Aligarh Muslim University, Aligarh-202002, Uttar Pradesh, India.
2Department of Basic Sciences, A. K. Tibbiya College; Aligarh Muslim University, Aligarh-202002, Uttar Pradesh, India.
- *Corresponding Author:
- Riaz Ahmad
Section of Genetics, Department of Zoology
Faculty of Life Science
Aligarh Muslim University
Aligarh 202002 (UP), India
Tel: 00919927018812
Fax: 00915712702885
E-mail: riazzool@rediffmail.com
Accepted date: April 21 2007
Polyacrylamide gel electrophoresis (PAGE) revealed that almost all of serum proteins of spotted snakehead Channa punctata precipitate at 75% ammonium sulphate (AS) saturation. AS fractions of 65% predominantly contained γ-globulins, traces of haptoglobin and β-globulin. Two of β-globulins, haptoglobins and transferrins were identifiable due to specific staining. Fraction of 45% AS saturation had only globulins, while fraction of 25% contained almost pure albumin-like protein. Albumin-like protein showed cross reactivity against rabbit anti-HSA antiserum. For albumin-like protein of Channa punctata, a molecular weight of 70 kD was determined by SDS-PAGE, which was similar to the value reported for HSA. Since serum albumin is not ubiquitously distributed in class Pisces, its occurrence in C. punctata, which is a fish of evolutionarily remote origin is significant.
Keywords
Serum proteins, albumin-like protein, ammonium sulphate fractionation, Channa punctata, PAGE.
Introduction
In addition to globulin and albumin like components that constitute approximately 60-97% of total serum proteins [1-2], other low concentrations or trace metabolites also exist in human serum. A recent proteomic study has identified up to 81 components in human plasma [3]. However, investigators generally relied on electrophoretic comigration of components of sera under investigation with well-characterized fractions of mammalian serum [4-6]. While due to prime role in immune response, much of the recent attempts on fish plasma proteins continue to focus on immunoglobulin [6-7], reports on identification and characterization of fish serum albumin are scanty [4,8-9]. Of the β-globulin group, polymorphic pure transferrins of Channa punctata had been identified further by several biochemical criteria [10].
From evolutionary perspective, quantitative and qualitative status of several well identified abundant proteins in lower vertebrates needs explanations. For instance, in some fish species albumin may exist in minute quantities, while sera of other fish species may entirely lack of it [11]. More primitive hagfish contain a large sized lipoprotein that binds molecules typically transported by vertebrate serum albumins [12]. Haptoglobin (Hp), that is traceable in class Pisces, is replaced by a protein PIT54 in reptiles and birds, while both Hp and PIT54 exist in ostriches [13]. Hp as the hemoglobin binding protein again appears in mammals.
In view of persistent significance of transferrin, albumin and immunoglobulins in genetics and pathogeneicity as well as their evolutionary implications, we have been interested to extend our study in identification and distribution of serum proteins of C. punctata. Our report describes the distribution and identification of haptoglobins and occurrence of albumin which were partially purified by ammonium sulfate (AS) fractions.
Materials and Methods
Live samples of spotted snakehead Channa punctata (Bloch) were brought to laboratory from the local fish market of Aligarh. Blood of each specimen was collected by cardiac puncture with a 2ml sterilized disposable syringe. Following clotting at room temperature serum that oozes out of the clot was collected in 1.5ml sterilized eppendorff tubes. Blood cells from the sera samples were removed by repeated centrifugation at a speed of 1174×g for 10 min [14]. Pure sera samples were analyzed afresh or stored at -20°C.
Protein contents were estimated according to the method of Lowry et al. [15].
Sera samples with resembling PAGE profiles (procedure outlined in the next paragraph) were pooled. Standard protocol of ammonium sulphate saturation was followed [16] to obtain different fractions of sera proteins. Four concentrations 25%, 45%, 65%, and 75% (w/v) of ammonium sulphate were selected for fractionation of pooled fish sera at 0°C, respectively.
Precipitates obtained following low speed centrifugation (2000 ×g) at 4°C were extensively dialyzed against chilled distilled water. In a final step of dialysis water was replaced with cold Tris-HCl buffer (25 mM; pH, 7.4).
Equal amounts of sera collected from different individuals, were loaded on gels lacking SDS for screening. Polyacrylamide gel electrophoresis (PAGE) was performed following the procedure of Laemmli [17] with SDS while to modify for native PAGE the detergent was skipped. After extensive dialysis to remove AS, 6-8μl (~15μg) of each cut was loaded on 7.5% polyacrylamide (PA) gels. Electrophoretic runs were made in vertical slabs (100×80×1mm) at 100V, 15mA and 1W up to 6 hrs and at 200V, 35mA and 1W up to 2 h for non-denaturing (native) and denaturing gels, respectively.
Non-denaturing gels were directly stained with Coomassie Brilliant Blue R-250 for 15 min and destained in 7% acetic acid, while denaturing gels were kept over night in 5% acetic acid to wash out SDS before staining. Identification of specific major sera proteins of C. punctata were also performed using specific staining of the gels as described earlier [18-20]. They were photographed on ORWO black and white negative film under transilluminator. The gel scans were used for software analyses.
Haptoglobins were identified by the method of Topchy [20], wherein 1g Fuschin acid was dissolved in 100 ml of 2% glacial acetic acid in the presence of 10g zinc sulphate. The working solution was prepared by adding 1ml of 3% hydrogen peroxide.
SDS-PAGE (10%) was carried out essentially according to the protocol of Laemmli [17]. Transfer of protein bands from SDS-PA gels onto PVDF membranes was performed at 150V, 30mA and 1W in transfer buffer containing Tris-glycine (Tris, 0.024M; Glycine, 0.19M), methanol (20%) and SDS (0.03%) at final concentrations. Immunocross reactivity was detected using rabbit anti-HSA serum as the primary source. As a secondary antisera Goat anti-IgG coupled with HRP (Horse Radish Peroxidase) was used and the cross reactivity signals were developed with LumiGLO chemiluminiscent kit.
Densitometric analysis and the molecular weight estimation of the PA gel scans were carried out using Scion Imaging and GelPro software programs, respectively.
Results
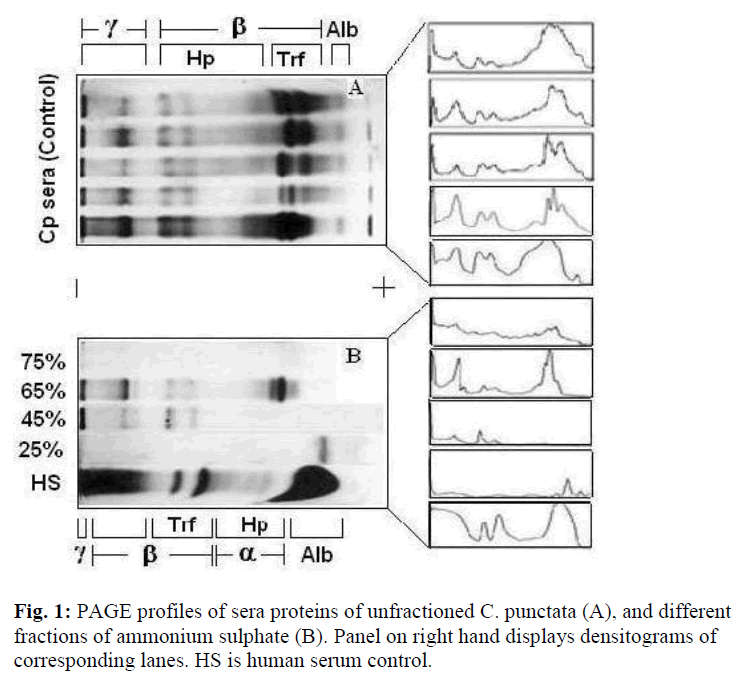
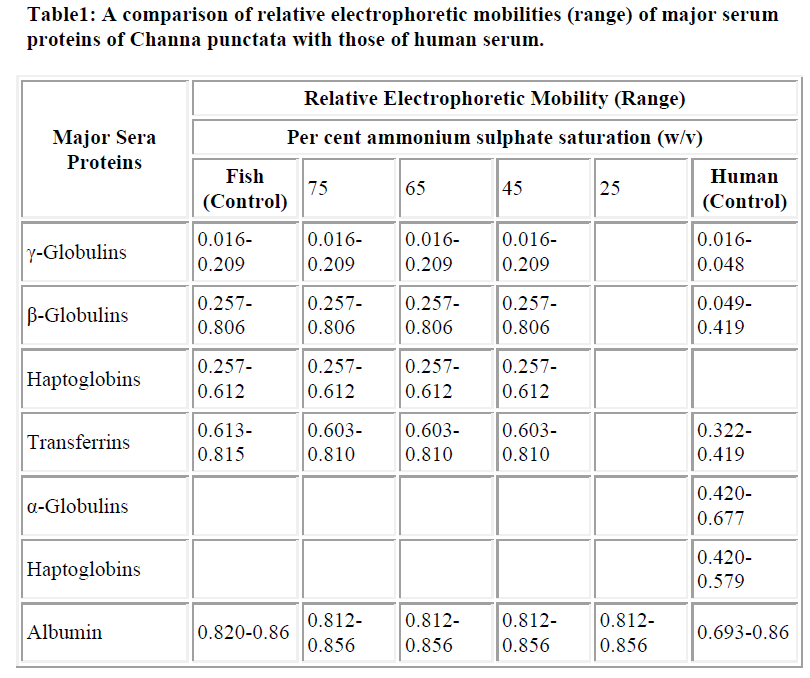
The total number of protein bands in PAGE profiles of C. punctata unfractioned serum (control) was visually counted as seventeen (Fig. 1A). Overloading obscured clear resolution of fastest migrating β-globulin bands (Fig. 1A, lanes 1,4 and 5) labeled as transferrins (Trf), while that labeled as albumin (Alb) was not visualized if the quantity of loaded serum was too low (Fig. 1A, lane-2). The unlabelled band that stacks at the buffer line may be prealbumin. However, no additional characterization of either this stack or minor bands or in the range of labeled as α-globulins was carried out. Differences in the relative amounts and electrophoretic mobilities of α, β and γ globulins of C. punctata and human serum (Fig. 1B, HS) were obvious. A summary of these differences is presented in Table- 1. The two most species specific differences are: exceptionally low amount of albumin-like protein in C. punctata and low quantity of Trf in human.
Under the conditions applied in this study almost all proteins precipitated out at 75% ammonium sulphate (AS) saturation (Fig.1B, top lane). Predominant proteins in 65% AS fraction were slowest migrating band of γ and fastest of β globulins (Fig. 1B, lane-2). Maximum yield of γ globulins was observed in PAGE patterns of 45% fraction (Fig. 1B, lane-3). Almost pure albumin like protein appeared in 25% fraction of fish sera that comigrated as a single sharp peak with the albumin band of unfractioned sera (Fig. 1B, lane-4). Densitometric tracings reproduced the quantitative and qualitative distribution of various proteins as bands in PAGE profiles of human and sera and its AS fractions (Fig.1, right hand panel).
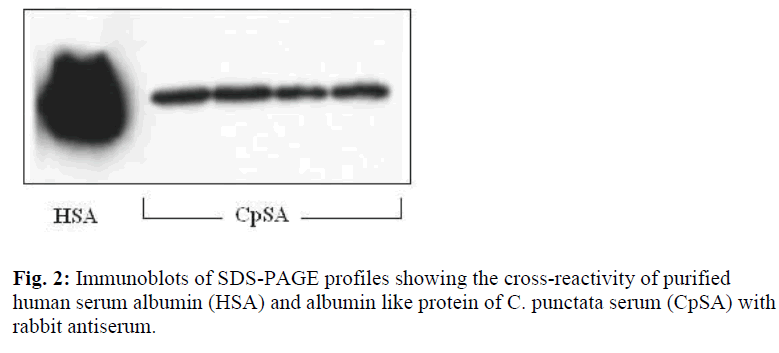
Of major proteins of C. punctata, haptoglobin was identified by staining that specifically develops protein hemoglobin complexes [20]. In immunoblots of SDS polyacrylamide gels, albumin like protein of C. punctata stacked as a band of molecular weight of ~70 kD that gave sufficiently strong signals with rabbit anti-HSA serum (Fig. 2). For identifying transferrins and γ globulins our previous studies (taken up under Discussion) were used as the guide.
Discussion
In this study, we fractioned sera of C. punctata at pH of 6.8 and 75%, 65%, 45% and 25% saturations of ammonium sulfate (AS). To minimize effect of seasonal and physiological variations [21], sera of resembling PAGE profiles were pooled for ammonium sulfate fractionation.
As described under results, the total number of bands was in the range previously reported for other teleost sera [5,21]. The number of countable bands depended on the amount of loaded sera. While some of the high concentration bands merge upon overloading, minor bands stacked more prominently (Fig. 1A, lanes 1 and 4). Albumin like band was not detected at low quantity loadings (Fig. 1A, Lane-4). Nomenclature γ, β and α globulins in PAGE profiles of C. punctata were based on four criteria : (i), the conventional increasing order of relative electrophoretic mobilities; (ii), solubility differences between albumin and globulins; (iii), co-precipitation of globulins (γ, β and α) and (iv), localization and identification of albumin like protein, transferrins and haptoglobins (Table-1, Fig. 1). Identification of albumin like protein by immunoblotting and of Hp by staining protocol of Topchy [20] is diagnostic. This suggests that relative positioning of globu lins in PAGE profiles of C. punctata and human sera are species specific. TF polymorphs of C. punctata have already been biochemically and functionally characterized in our laboratory [10], while γ globulin bands have been previously identified by Shafeeq [6].
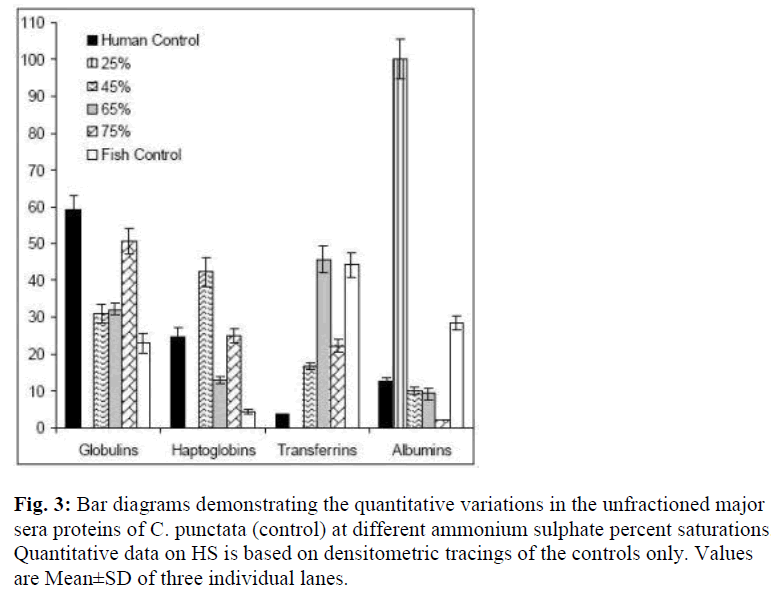
Co precipitation of slowest migrating γ bands and TF (β globulins) at 65% of AS saturation suggests resembling state of surface hydrophilic amino acid residues, which determines solubility under changed ionic strengths or the pH. Obviously, another β globulin, Hp undergoes such a change in state of solubility at 45% AS and may be exploited as first purification step. Low salt solubility requirement of albumin like band is typical of this class of protein. The quantitative differences in the levels of γ, β and α globulins of C. punctata were more remarkable if compared with these proteins of human serum (Fig. 3). While albumin was the major protein of human sera, its comigrating band existed in traces in C. punctata serum. In contrast, concentration of TF was substantially high in sera of this fish (Fig. 1 and 3, Table-1). These differences were also species specific.
Figure 3: Bar diagrams demonstrating the quantitative variations in the unfractioned major sera proteins of C. punctata (control) at different ammonium sulphate percent saturations. Quantitative data on HS is based on densitometric tracings of the controls only. Values are Mean±SD of three individual lanes.
Transferrin (TF), which is a β globulin occurred in high concentration in C. punctata not only binds and transport iron, but restricts bacterial infections by keeping the free iron concentration low. Interaction of TF with bacteria is being currently looked upon as the principal factor of natural selection in evolution of teleosts [22]. Interestingly, another globulin Hp that normally binds free hemoglobin in plasma has recently been found to form complexes, which possess trypanolytic activity in human sera [23]. In view of an unusual role of fish TF in controlling pathogeneicity, extra significance of fish Hp in defense related mechanisms can not be ruled out. More efforts are therefore required to investigate interaction of fish serum proteins and to characterize them functionally and structurally.
The presence of albumin in fishes is not ubiquitous. This report is the first on albumin like protein of C. punctata. Albumin like protein of its sister species C. gachua, also reported from this laboratory was found to be dimorphic [9]. This information on albumin genetics helped in identifying ulcerative disease prone strains of C. gachua. It is significant that albumin like protein exists in at least two of channids, which are a specialized group of remote evolutionary origin. The primitive hagfish has been reported to lack albumin but it is absent in advanced teleosts such as Cyprinus carpio [11]. In rainbow trout, where albumin is better characterized, it is a major protein that binds bromocresol blue [4]. Similarly, African catfish (Clarias gariepinus) serum albumin (CfSA) binds bilirubin [8]. Albuminlike protein of C. punctata and CfSA shares the characteristics of cross-reactivity with rabbit anti-HSA antiserum (Fig. 1B).
There is no evidence yet to show that fish serum proteins perform general functions other than assigned to their mammalian counterparts. This includes keeping the oncotic pressure, storage and transport of numerous metabolites and the defense [10]. Novel proteins with specific function may, however, exist. Examples are antifreeze glycoprotein in Antarctic fish sera [24] and Tributyltinbinding protein in Japanese flounder [25].
In conclusion, the results presented here demonstrate the presence of albumin like protein and haptoglobin in C. punctata. The fish serum has transferrin in unusually high quantities. Relative amounts, solubility and electrophoretic mobilities of serum proteins of C. punctata suggest some homology with those of human serum. There are, however, outstanding species specific differences in the solubility, relative electrophoretic mobilities and quantities of the identified components. As published information on sera of Indian fish species is quite inadequate, the data on identification of serum components needs to be expanded.
Acknowledgements
Authors are grateful to the University for providing the facilities for this work. Thanks are also due to Dr. A.L. Bilgrami (Rutgers University, USA) for his help. Financial assistance to one of us (RA) in the form of University fellowship is also thankfully acknowledged.
References
- Ahmed N, Barker G, Oliva K, Garfin D, Talmadge K, Georgiou H, Quinn M, Rica G. An approach to remove albumin for the proteomic analysis of low abundance biomarkers in human serum. Proteomics 2003; 3: 1980-1987.
- Chen YY, Lin SY, Yeh YY, Wu CY, Chen ST, Wang AH. A modified protein precipitation procedure for efficient removal of albumin from serum. Electrophoresis 2005; 26: 2117-2127.
- Park GW, Kwon KH, Kim JY, Lee JH, Yun SH, Kim SI, Park YM, Cho SY, Paik YK, Yoo JS. Human plasma proteome analysis by reversed sequence database search and molecular weight correlation based on a bacterial proteome analysis. Proteomics 2006; 6: 1121–1132.
- Maillou J, Nimmo IA. Albumin like proteins in the serum of Rainbow trout (Salmo gairdneri). Comp Bio-chem Physiol 1993; 104B: 387-393.
- Thurston RV. Electrophoretic patterns of blood serum proteins of from rainbow trout, Salmo gairdneri. J Fish Res Bd Can 1967; 24: 2169-2188.
- Shafeeq TY. Characterization of antibodies in selected freshwater teleostean fish species. M Phil submitted to Aligarh Muslim University, Aligarh. 1997.
- Scapigliati G, Chausson F, Cooper EL, Scalia D, Mazz-ini M. Qualitative and quantitative analysis of serum immunoglobulins of four Antarctic fish species. Polar Biol 1997; 18: 209-213.
- Hasnain A, Arif SH, Ahmad R, Jabeen M, Khan MM. Biochemical characterization of a protein of albumin multigene family from serum of African catfish Clarias gariepinus Bloch. Ind J Biochem Biophys 2004; 41: 148-153.
- Jabeen M, Hasnain A. Identification of an ulcerative disease sensitive phenotype of Channa gachua with the help of serum albumin polymorphism. Proc Nat Symp Fish Health Manag, GB Pant Univ Agricult Technol, Pantnagar, India, 2001, 149-154.
- in A, Nabi N. Partial biochemical characterization and ironHasna binding capacities of transferrin variants of air breathing murrel Channa punctata. Asian Fish Sci (2007). In press
- De Smet H, Blust R, Moens L. Absence of albumin in the plasma of common carp Cyprinus carpio: binding of fatty acids to high density lipoprotein. Fish Physiol Biochem 1998; 19: 71-81.
- Gray JE, Doolittle RF. Characterization, primary structure, and evolution of lamprey plasma albumin. Protein Sci 1992; 1: 289-302.
- Wicher KB, Fries E. Haptoglobin, a hemoglobin binding protein, is present in bony fish and mammals but not in frog and chicken. Proc Nat Acad Sci, USA 2006; 103: 4168-4173.
- Holler L, Van der Laarse A. Interference of the measurement of lactate dehydrogenase (LDH) activity in human serum and plasma by LDH from blood cells. Clin Chim Acta 1979; 99: 135-142.
- Lowry OH, Rosenbrough HJ, Farr AL, Randall RS. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265-275.
- Dawson RMC, Elliott DC, Elliott WH, Jones K. Data for Biochemical Research. 2nd Ed Oxford University Press 1969. pp 615-616.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680-685.
- Hasnain A, Ali SA, Khan IA. Transferrin polymorphism in Channa punctatus. Curr Sci 1981; 50: 511.
- Nabi N, Arif SH, Hasnain A. Genetic structure of natural populations of Channa punctatus Bloch in the Rohilkhand plains of India. Asian Fish Sci 2003; 16: 77-84.
- Topchy GL. On the method of staining haptoglobin on the electropherograms. Cyto Gen 1970; 277-278.
- Samantaray K, Das AB. Seasonal variations in the electrophoretic patterns of serum proteins of Channa punctatus Bl. J Aqua 1995; 3: 9-11.
- Ford MJ. Molecular evolution of transferrin: Evidence for positive selection in salmonids. Mol Biol Evol 2001; 18: 639-647.
- Nakajima K, Moestrup SK, Pays E, Vanhollebeke B., Nielsen MJ, Watanabe Y, Truc P, Vanhamme L. Distinct role of haptoglobin related protein and apolipoprotein L-1 in trypanolysis by human serum. Proc Nat Acad Sci, USA, 2007; 104 : 4118-4123.
- Feeney RE and Osuga D. Comparative biochemistry of Anatarctic proteins. Comp Biochem Physiol 1976; 54A: 281-286.
- Shimasaki Y, Yuji S, Oshima Y, Yokota Y, Kitano T, Nakano KM, Kawabata S, Imada N, Honjo T. Purfication and identification of a tributyltin-binding protein from serum of Japanese flounder, Paralychthys olivaceous. Environ Toxicol Chem 2002; 21: 1229-1235.