ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Artificial Intelligent Techniques for Bio Medical Signal Processing: Edition-I
Bone marrow mesenchymal stem cells overexpressing BFGF mediated the repair of chronic obstructive pulmonary disease in a rat model
Shanshan Peng1#, Liangying Zhong2#, Yue Zhao1, Huala Wu1, Lin Ma1, Guilan Wen1, Wei Zhang1 and Xin Gan1*
1Department of Respiratory Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, PR China
2Department of Clinical Laboratory, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong Province, PR China
#Shanshan Peng and Liangying Zhong contributed equally to this article.
- *Corresponding Author:
- Xin Gan
Department of Respiratory Medicine
First Affiliated Hospital, Nanchang University, PR China
Accepted date: January 09, 2017
Objectives: To explore the role of basic Fibroblast Growth Factor (bFGF) overexpression in bone marrow mesenchymal stem cells on the repair of chronic obstructive pulmonary disease in rats.
Result: We found that the engrafted Bone Marrow Mesenchyme Stem Cells (BMSCs) survived in the rat lung tissues. The Oxygen Partial Pressure (PO2) and Blood Oxygen Saturation (SaO2) values in the Protein C (PC) group, M group, P group, and B group were lower than those in the Nucleated Cells (NC) group. The number of White Blood Cells (WBC) and Neutrophils (N) in the NC, M, P, and B groups were significantly lower than in the PC group. Emphysema was observed in the PC, M, P, and B groups. The MAN, MAA and MLI values in the M, P and B groups were improved compared to the PC group.
Conclusions: Our findings revealed that transplantation therapy using BMSCs overexpressing bFGF significantly improved the symptoms of the repair effect compared to the BMSC transplantation group in a rat model of Chronic Obstructive Pulmonary Disease (COPD).
Keywords
Mesenchymal stem cell, bFGF, BMSCs.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a common disease worldwide. There are approximately 200 million COPD patients in the world, and approximately 3 million people die of this disease each year [1]. In recent years, as environmental pollution has become more serious and more women have taken up smoking, its incidence has increased year by year. Currently, COPD is a major cause of morbidity and mortality and the fourth leading cause of death. COPD is characterized by limited airflow that is persistent but not completely reversible. Limited airflow exhibits progressive development and induces characteristic pathological changes in the central airway, peripheral airway, lung parenchyma, and pulmonary vessels. After damage, the body cannot completely repair the organ; therefore, COPD results in irreversible lesions [2]. With the exception of oxygen therapy, which has been shown to improve arterial hypoxemia in a small subset of smoking cessation patients, there have been few other treatments for COPD that prolong patient survival time, and no treatment method can provide a full recovery in patients with impaired lung function. If we could find an effective method that could repair the injured lung tissue and reconstruct pulmonary lobules, it would play an important role in the clinical treatment of COPD.
Mesenchymal Stem Cells (MSCs), which are derived from mesodermal stem cells, exhibit multi-directional differentiation ability and strong plasticity. Under certain conditions, MSCs can differentiate into a variety of sources of mesoderm cells, such as bone, cartilage, muscle cells, and fat cells; they can also differentiate into neurons, liver and biliary cells, myocardial cells, renal tubular epithelial cells, lung epithelial cells, and islet cells, among other cell types. MSCs can suppress the proliferation of allogeneic T cells and can escape immune recognition in patients with normal immunity due to low expression of Human Leucocyte Antigen (HLA) and a strong immunosuppressive effect [3,4]. Bone Marrow Mesenchymal Stem Cells (BMSCs) are primitive cells that can differentiate into many types of tissue cells for clinical use. Gradually, as more in-depth research on BMSCs has accumulated, the opinion that BMSCs can reach injured lung tissue and differentiate into alveolar epithelial cells in the injured lung tissue has been accepted [5,6].
Studies show that basic Fibroblast Growth Factor (bFGF) promotes angiogenesis [3,4,7] and regulates MSCs to differentiate into vascular endothelial cells. If we could overexpress bFGF in the lung tissue, it could induce migration of MSCs to differentiate into endothelial cells and promote alveolar capillary regeneration, improving the alveolar and functional defect resulting from ischemia and thus achieve the effect of repairing COPD lung tissue. Because of the short half-life of exogenous bFGF and because the autocrine ability of lung tissues is limited, it is necessary to find a suitable carrier that can transport bFGF into the body and engraft in the lung tissue to express bFGF stably and robustly.
Materials and Methods
In this study, we used rat BMSCs as a bFGF gene carrier for expressing bFGF stably and robustly in lung tissue. We determined the influence of the engrafted BMSCs overexpressing bFGF on lung tissue pathology, inflammatory cells and blood gas assay in COPD rats. We hope to illuminate the repair function of bFGF-overexpressing BMSCs in COPD rats and to provide an experimental basis for further research.
Isolation and culture of BMSCs
Sprague-Dawley (SD) rats were provided by the Experimental Animal Center of the Nanchang University School of Medicine. Four- to five-week-old SD rats (male or female) were executed by cervical dislocation. We then removed and separated the femur and tibia, then flushed the medullary cavity with pre-warmed Phosphate-Buffered Saline (PBS) repeatedly until the medullary cavity turned white. The rinses were collected into sterile centrifuge tubes. The cells were resuspended in low-sugar Dulbecco’s Modified Eagle’s Media (DMEM) containing 10% Fetal Bovine Serum (FBS) after centrifugation (complete medium) and then seeded into a 25 cm2 flask and incubated at 37°C and 5% CO2. When the cell confluence reached 80-90%, the cells were digested with 0.25% trypsin containing 0.125% Ethylene Diamine Tetraacetic Acid (EDTA) and resuspended with complete culture after centrifugation and divided into two flasks.
Flow cytometry analysis
BMSCs at P3 were digested and separated into three tubes. 0.02 mL each of Cluster of Differentiation (CD) 44- Fluorescein IsoThioCyanate (FITC), CD29-FITC and CD34- PE antibodies (all from BD Biosciences, San Jose, CA, USA) were added to the 3 tubes, and the tubes were incubated at room temperature in the dark for 30 min. Then, the cells were washed with PBS and resuspended to obtain single cells. The samples were tested using the flow cytometry instrument (BD Biosciences) and data were analyzed with Fetal Calf Serum (FCS) Express V3 software.
Determination of screening concentration of hygromycin
The BMSCs of passage 3 were seeded into 24-well plates, cultured at constant temperature in an incubator with 37°C and 5% CO2. The cell culture medium of the first row was replaced by medium with hygromycin (Life Technologies, Grand Island, NY, USA) 24 h later, and the hygromycin concentration gradient was 15, 20, 25, 30, 35 and 40 μg/mL. The cell culture medium of the second to fourth rows was replaced with complete medium without hygromycin as control. The culture medium was replaced every 3 to 5 days. The cell survival rate of each well with or without hygromycin was checked 2 weeks later, and the lowest concentration that killed all the cells was considered the minimum effective concentration.
Plasmid construction
E. coli DH5α was obtained from the clinical laboratory in the First Affiliated Hospital of Nanchang University. bFGFpcDNA3.1 and pcDNA3.1 plasmids were purchased from and sequenced by Invitrogen. The double enzyme method was used to construct the recombinant plasmid bFGF-pcDNA3.1. As described by the manual of the OMEGA plasmid extraction kit (Omega Bio-tek, Norcross, GA, USA), we extracted and purified bFGF-pcDNA3.1 from DH5 bacteria with eukaryotic expression vector containing bFGF-pcDNA3.1 for enzyme digestion and sequencing identification.
Transfection of bFGF gene into BMSCs
The bFGF gene was transfected into BMSCs by liposome transfection. BMSCs of P3 generation were plated in a cell culture dish, the medium was replaced by serum-free medium (DMEM with double PS) 24 h later. Then, the serum-free medium was replaced with transfection medium that contained Lipofectamine 2000 (Life Technologies) and the bFGF plasmid (l250 μL Opti-MEM + 20 μg bFGF plasmid) + (1250 μL Opti- MEM + 50 μL Lipofectamine 2000) 3 h later. After 5 h, the transfection medium was replaced with complete medium. After transfection for 48 h, the medium was replaced with complete medium containing 20 μg/mL hygromycin. Fourteen days after the treatment, the surviving cells were the cells overexpressing bFGF. The transfection efficiency was detected by measuring green fluorescent protein expression.
Preparation of COPD rats
We established the rat COPD model using smoke combined with LPS stimulation. On the first and fourth days, 200 μL LPS (1 mg/mL) was injected into each rat trachea. The rats were smoked (60 min each time, once a day, five cigarettes) in a closed box from day 2 to day 13, and day 15 to day 28. Fifty healthy SD rats at 8 weeks of age, both male and female, weighing approximately 180-200 g, were randomly divided into the following five groups and started the following treatments: normal control group (NC, n=10): injecting 3 mL PBS solution in the tail vein; COPD control group (PC, n=10): set up COPD models and immediately injecting 3 mL PBS solution in the tail vein; MSCs treatment group (M, n=10): after building COPD models and immediately injecting the third generation of BMSCs 1 × 107/3 mL PBS solution in the tail vein; pcDNA3.1-MSCs treatment group (P, n=10): after building COPD models and immediately injecting the third generation of BMSCs 1 × 107/3 mL PBS solution with transacting pcDNA3.1 plasmid in the tail vein; bFGF - pcDNA3.1 MSCs treatment group (B, n=10): after building COPD models and immediately injecting the third generation of BMSCs 1 × 107/3 mL PBS solution with transacting bFGFpcDNA3.1 plasmid in the tail vein.
Transplantation and physiological index detection
bFGF-pcDNA3.1 BMSCs transplanted into the rat model by tail intravenous injection and extracted with uniform handling after 30 days. The arterial blood of rats was harvested and tested for blood gas assay using a blood gas analyzer (Radiometer Medical ApS, Copenhagen, Denmark). The lung tissue was processed into frozen sections and paraffin sections to observe the engrafting of BMSCs in the lung tissue under the fluorescence microscope and to observe morphological changes under the microscope after H&E staining. The Bronchodilator Lavage Fluid (BALF) was collected, and the percentages of White Blood Cells (WBC) and neutrophils (N %) were counted.
Conditioned Media (CM)-Dil staining
BMSCs were stained with CM and Dil (Life Technologies) at 37°C for 5 min and 4°C for 15 min and then observed using an inverted fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
The data (mean ± SE) were analyzed with statistical software SPSS 11.5 to compare multiple sets of variance analysis and comparison using the Student-Newman-Keuls (SNK) method between the groups, a P<0.05 was regarded as statistically significant.
Results
Isolation and identification of rat BMSCs in vitro
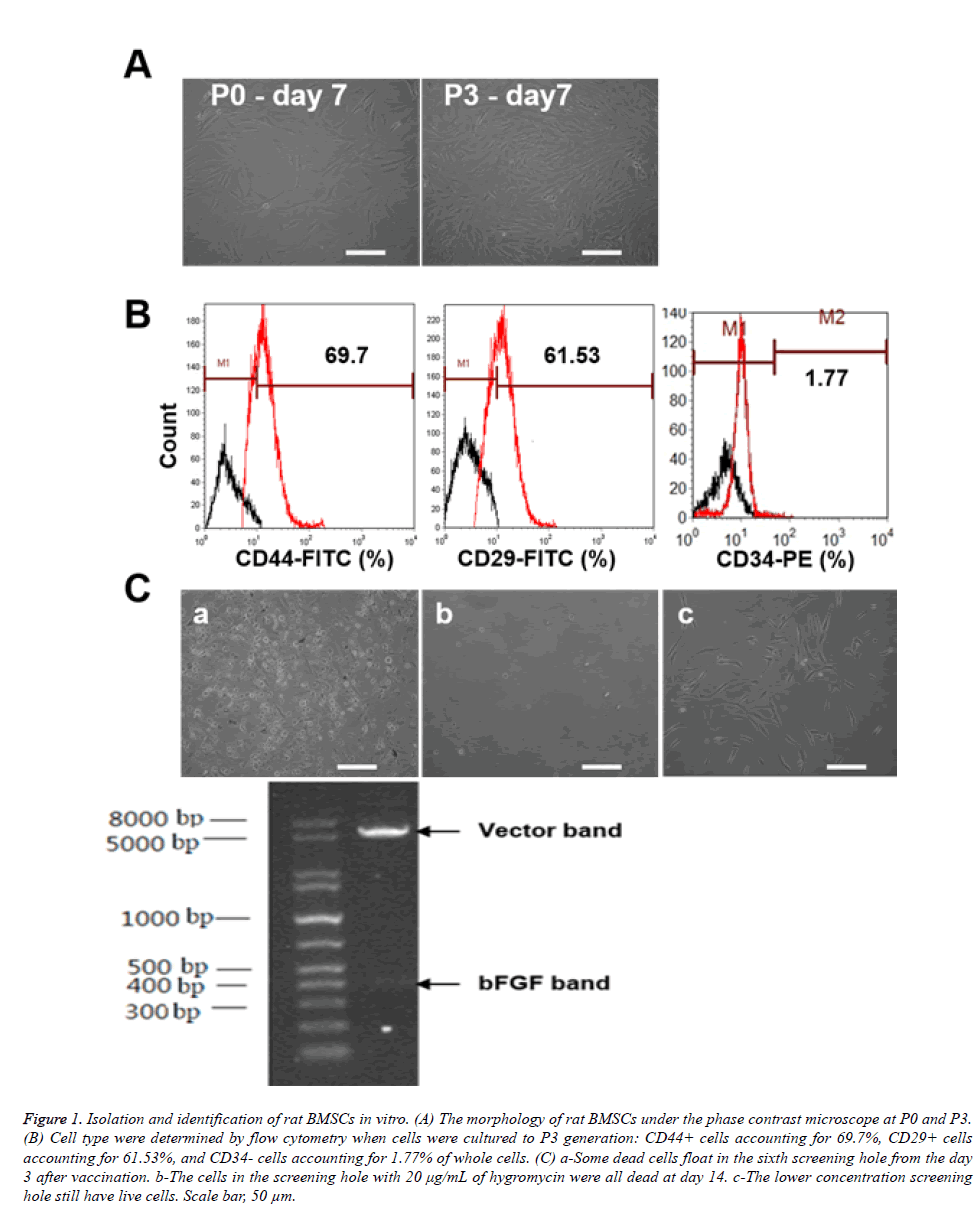
Primary rat BMSCs were isolated and cultured in 25 cm2 bottles. Adherent cells increased at day 3 and emerged as fusiform, triangular or spindle-shaped BMSC colonies. The nuclear mass ratio was large, and small colonies formed locally. After the cells reached 85-90% confluence at day 7-10 (Figure 1A) and had an appearance of fish, spiral or radial alignment, they were considered ready for passage. The passaged cells grew more quickly than the primary cells, kept primary morphology, and reached confluence with more uniform morphology after another 3-5 days (Figure 1A). Cell type was determined by flow cytometry when cells were cultured at the P3 generation (Figure 1B): CD44-positive cells accounting for 69.7%, CD29-positive cells accounting for 61.53%, and CD34 negative cells accounting for 1.77% of all cells. These results illustrated that the isolated cells were BMSCs. We found some dead cells floating in the sixth screening well beginning at day 3 after vaccination (Figure 1C), and the number of dead cells was significantly positively correlated with the concentration of hygromycin. On day 14, we could see that the cells in the screening well with 20 μg/mL of hygromycin were all dead (Figure 1C), while the lower concentration screening well still contained live cells (Figure 1C). Therefore, we concluded that the minimum effective concentration of hygromycin to screen BMSCs of rats was 20 μg/mL.
Figure 1: Isolation and identification of rat BMSCs in vitro. (A) The morphology of rat BMSCs under the phase contrast microscope at P0 and P3. (B) Cell type were determined by flow cytometry when cells were cultured to P3 generation: CD44+ cells accounting for 69.7%, CD29+ cells accounting for 61.53%, and CD34- cells accounting for 1.77% of whole cells. (C) a-Some dead cells float in the sixth screening hole from the day 3 after vaccination. b-The cells in the screening hole with 20 μg/mL of hygromycin were all dead at day 14. c-The lower concentration screening hole still have live cells. Scale bar, 50 μm.
Establishment of a line of BMSCs overexpressing bFGF
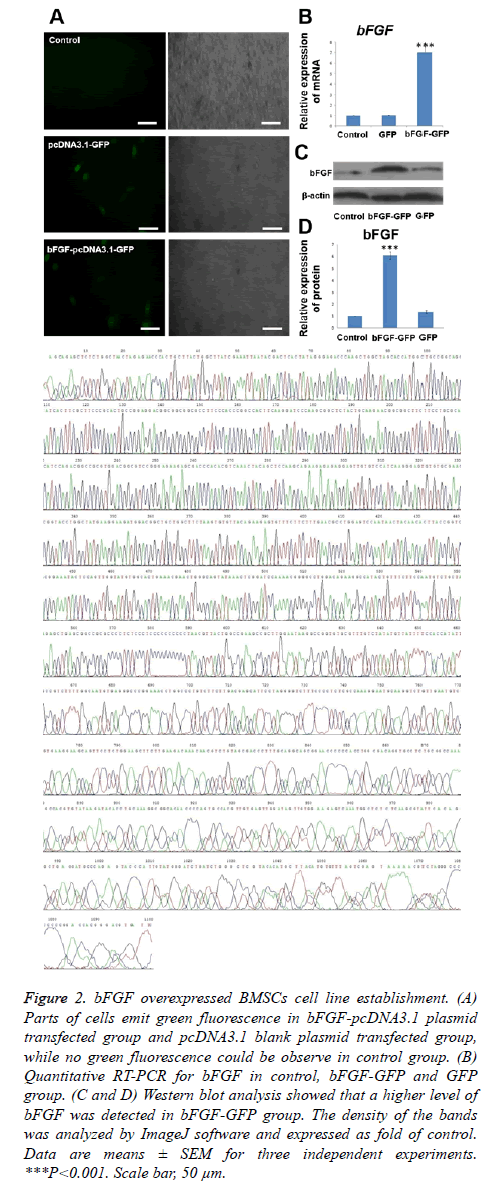
After transfection for 48 h, we observed the cells under an inverted fluorescence microscope. Portions of the cells emit green fluorescence (Figure 2A) because the bFGF-pcDNA3.1 plasmid and pcDNA3.1 blank plasmid both carry the GFP gene. This observation proved that the transfection was successful and green fluorescent protein was expressed. The transfection efficiency for both plasmids was determined to be approximately 50%. We evaluated the bFGF mRNA and protein expression levels of BMSCs transfected after 48 h (Figures 2B-2D). The expression of the bFGF gene could be detected in the cells of the three groups, and there was a statistically significant difference (p<0.001) in the expression of the bFGF gene between the bFGF-pcDNA3.1 plasmid transfection group with blank pcDNA3.1 plasmid transfection and the non-transfected group, while there was no significant difference between the pcDNA3.1 plasmid transfection group and the non-transfected group (p>0.05). This showed that the expression of the bFGF gene was high in the bFGF-pcDNA3.1 plasmid transfection group.
Figure 2: bFGF overexpressed BMSCs cell line establishment. (A) Parts of cells emit green fluorescence in bFGF-pcDNA3.1 plasmid transfected group and pcDNA3.1 blank plasmid transfected group, while no green fluorescence could be observe in control group. (B) Quantitative RT-PCR for bFGF in control, bFGF-GFP and GFP group. (C and D) Western blot analysis showed that a higher level of bFGF was detected in bFGF-GFP group. The density of the bands was analyzed by ImageJ software and expressed as fold of control. Data are means ± SEM for three independent experiments. ***P<0.001. Scale bar, 50 μm.
Distribution and location of BMSCs in lung tissue of rats
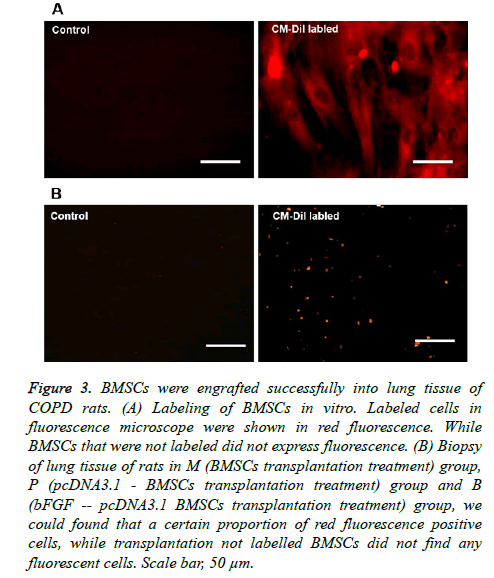
The BMSCs of the transfection group and the non-transfected group were both labeled with CM-Dil in vitro; labeled cells exhibited red fluorescence under the fluorescence microscope. While BMSCs that were not labeled did not express fluorescence (Figure 3A), the labeling rate was above 95%. The labeled BMSCs were all transplanted into rats, and rats were executed after 30 days. Under the fluorescence microscope, in the biopsy of lung tissue of rats in the M (BMSC transplantation treatment) group, P (pcDNA3.1 - BMSC transplantation treatment) group and B (bFGF - pcDNA3.1 BMSC transplantation treatment) group, we found a certain proportion of red fluorescence-positive cells, while we did not find any fluorescent cells after transplantation of unlabeled BMSCs (Figure 3B). These results showed that BMSCs were engrafted successfully into the lung tissue of COPD rats.
Figure 3: BMSCs were engrafted successfully into lung tissue of COPD rats. (A) Labeling of BMSCs in vitro. Labeled cells in fluorescence microscope were shown in red fluorescence. While BMSCs that were not labeled did not express fluorescence. (B) Biopsy of lung tissue of rats in M (BMSCs transplantation treatment) group, P (pcDNA3.1 - BMSCs transplantation treatment) group and B (bFGF -- pcDNA3.1 BMSCs transplantation treatment) group, we could found that a certain proportion of red fluorescence positive cells, while transplantation not labelled BMSCs did not find any fluorescent cells. Scale bar, 50 μm.
The blood gas assay results of rats in each group after delivery
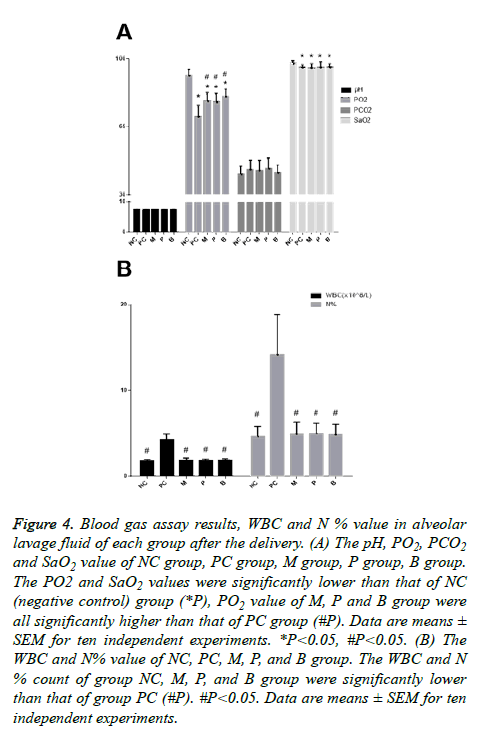
The blood gas assay showed that the PC (positive control) group, M group, P group and B group exhibited mild hypoxemia, the PO2 and SaO2 values were significantly lower than that of the NC (negative control) group (Figure 4A), the PO2 value of the M, P and B groups were all significantly higher than that of the PC group, and there were no statistically significant differences among the three groups. However, it appeared that the PO2 value of B group had a rising trend compared with the M group and the P group. There were no statistically significant differences in the SaO2 value among the PC, M, P and B groups.
Figure 4: Blood gas assay results, WBC and N % value in alveolar lavage fluid of each group after the delivery. (A) The pH, PO2, PCO2 and SaO2 value of NC group, PC group, M group, P group, B group. The PO2 and SaO2 values were significantly lower than that of NC (negative control) group (*P), PO2 value of M, P and B group were all significantly higher than that of PC group (#P). Data are means ± SEM for ten independent experiments. *P<0.05, #P<0.05. (B) The WBC and N% value of NC, PC, M, P, and B group. The WBC and N % count of group NC, M, P, and B group were significantly lower than that of group PC (#P). #P<0.05. Data are means ± SEM for ten independent experiments.
The results of WBC and N % in alveolar lavage fluid after treatment of each group of rats
The WBC and N% of the NC, M, P, and B groups were significantly lower than that of the PC group (Figure 4B). The analysis of variance difference was statistically significant. There was no statistically significant difference among the NC, M, P, and B groups, but we found that the WBC and N% counts in three treatment groups, group M, group P, and group B, were slightly higher than that of the NC group. There was no decreasing trend observed when comparing the WBC and N % count in group B with group M and group P.
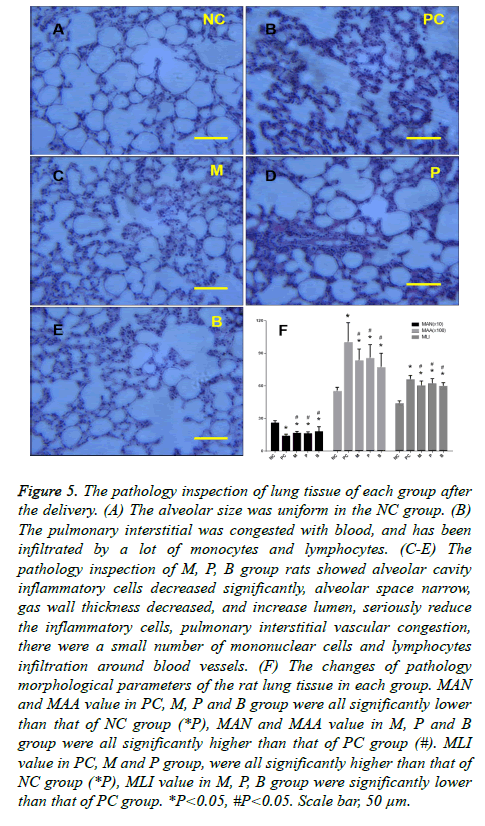
The pathology of lung tissue of each group after delivery
Inspection of the pathology of the lung tissue in the NC group showed that alveolar size was uniform. There were no obvious alveolar wall fractures and pulmonary bulla, and no obvious exudation of inflammatory cells was found. Airway wall thickness was normal. Inspection of the pathology of the inflammatory cells of the PC group rats revealed leakage of the alveolar space and expansion of the alveolar cavity, lung bullae rupture or pulmonary bullae fusion formation, airway wall thickening at different levels, hyperplasia of airway epithelial mucous glands and goblet cells, hypertrophy, luminal stenosis, wall neutrophils, lymphocytes, and infiltration with macrophages. The pulmonary interstitial was congested with blood and had been infiltrated by numerous monocytes and lymphocytes (Figure 5B), indicating bronchitis and emphysema. Inspection of the pathology of the M, P, and B group rats showed a significant decrease in alveolar cavity inflammatory cells, narrowing of the alveolar space, decreased gas wall thickness, increased lumen size, seriously reduced inflammatory cells, pulmonary interstitial vascular congestion, and infiltration by a small number of mononuclear cells and lymphocytes around the blood vessels (Figures 5C-5E). There was no significant difference among the M, P and B groups but compared with the M and P groups, there was a slightly higher degree of improvement observed in the B group. Figure 5F shows the pathological changes of morphological parameters of the rat lung tissue in each group. These results showed that the values of MAN and MAA in the PC, M, P and B groups were all lower than that of the NC group. The difference determined by the variance analysis was statistically significant. The values of MAN and MAA in the M, P and B groups were all higher than that of the PC group, and the difference was statistically significant, but there was no statistical significance among the three groups. A rising trend in the values of MAN and MAA in the B group was apparent when compared with the M and P groups. The values of MLI in the PC, M and P groups were all higher than that of the NC group. The difference by the variance analysis was statistically significant. The values of MLI in the M, P, and B groups were lower than that of the PC group, and the difference was statistically significant, but there was no statistically significant difference among these three groups. There was a slight decline in the value of MLI in group B compared with the M and P groups.
Figure 5: The pathology inspection of lung tissue of each group after the delivery. (A) The alveolar size was uniform in the NC group. (B) The pulmonary interstitial was congested with blood, and has been infiltrated by a lot of monocytes and lymphocytes. (C-E) The pathology inspection of M, P, B group rats showed alveolar cavity inflammatory cells decreased significantly, alveolar space narrow, gas wall thickness decreased, and increase lumen, seriously reduce the inflammatory cells, pulmonary interstitial vascular congestion, there were a small number of mononuclear cells and lymphocytes infiltration around blood vessels. (F) The changes of pathology morphological parameters of the rat lung tissue in each group. MAN and MAA value in PC, M, P and B group were all significantly lower than that of NC group (*P), MAN and MAA value in M, P and B group were all significantly higher than that of PC group (#). MLI value in PC, M and P group, were all significantly higher than that of NC group (*P), MLI value in M, P, B group were significantly lower than that of PC group. *P<0.05, #P<0.05. Scale bar, 50 μm.
Discussion
Chronic Obstructive Pulmonary Disease (COPD) is a common disease of the respiratory system, and it has very high morbidity and mortality. It will result in recurring Acute Exacerbation COPD (AECOPD) after a long course of the disease, eventually leading to respiratory failure and death even with treatment. In recent years, stem cell therapies and gene therapies based on stem cell technology have gained increasing attention, providing new approaches to tissue repair. In the in vitro culture system, under specific conditions, MSCs differentiate into neurons, islet cells, liver cells, renal tubular epithelial cells, and lung epithelial cells, among other types [5,6,8-11]. MSCs and the lung parenchyma cells differentiated from MSCs play a role in lung tissue repair [12,13]. Studies have shown that MSCs may have a special affinity for lung tissue [14]. This suggests gene therapy based on lung tissue repair by MSCs could be highly effective.
The most important aspect of transplantation of MSCs in the treatment of disease is that MSCs could colonize the damaged tissues. Orlic et al. found that MSCs can be recruited to the sites of injury by signals released from the damage tissue [15]. In animal models of tissue damage, the cytokines and chemokines secreted by the injured tissues and inflammation sites can recruit transplanted MSCs for tissue repair. This is an important theoretical basis for the transplantation of MSCs by intravenous delivery. Other studies have shown that a lower level of injury can induce autologous MSCs for tissue repair and in the context of a higher level of injury the autologous MSCs are not sufficient to promote tissue healing [16]. At this point, transplanting exogenous stem cells will play a critical role in tissue repair. Fischer et al. found that the majority of intravenously infused MSCs and BMSCs were embedded in the capillary network and stranded in the lungs [17,18], which is called the lung first effect [19]. This could be related to cell size, surface adhesion and the number of transplanted cells, which is consistent with our early findings. These results provide conditions for the venous transplantation of MSCs and BMSCs in the treatment of COPD lung injury diseases. After MSCs or BMSCs are stranded in the pulmonary capillary network, they could interact with the local microenvironment and surrounding tissue cells and gradually migrate to the airway and pulmonary vascular wall, pulmonary interstitial and alveoli for lung repair.
Basic Fibroblast Growth Factor (bFGF) is one of the most important members of the blood vessel growth factor family and is expressed mainly in the airway epithelial cells and basement membrane. bFGF plays an essential role in lung development [16]. Studies have shown that the presence of bFGF in the blood capillary in vitro, not only can promote cell proliferation, division and growth but can also induce the capillaries to form a cavity. Additionally, we found bFGF has a strong vascular chemotaxis effect in endothelial cells. By measuring peripheral Vascular Endothelial Growth Factor (VEGF) and the level of bFGF in COPD patients with an acute aggravating period, Pavlova et al. found that VEGF and bFGF could promote angiogenesis, increase pulmonary blood flow perfusion and improve tissue oxygenation [17]. By endotracheal bFGF treatment in a dog emphysema model induced by elastase, Marino et al. found that arterial blood oxygen partial pressure clearly increased after treatment [20,21]. In bFGF emphysema animal models, pathology observation revealed that pulmonary blood flow increased significantly, MLI decreased, and MAN clearly increased after treatment. These results showed that the degree of emphysema was improved and the lung lesions were repaired. We assessed the extent of the improvement of COPD treatment by transplantation with bFGF-BMSCs from several aspects, including blood gas assay, pathological change and alveolar lavage inflammatory cell count after transplantation of BMSCs in a rat model of COPD. We found that pure BMSC transplantation therapy, pcDNA3.1-BMSC transplantation therapy, and bFGF-pcDNA3.1 BMSC transplantation therapy improved hypoxemia in COPD rats, lung tissue pathology, and alveolar inflammation, but there was no significant difference between bFGF-pcDNA3.1 BMSC transplantation therapy and wild-type BMSC transplantation therapy, from the perspective of hypoxemia and lung tissue pathology. There was a trend toward improvement that suggested that the bFGF gene transacting in BMSCs in did not significantly affect COPD repair. This may be because transfection efficiency was too low to cause sufficient expression of bFGF or because changes in the microenvironment of the lung tissue in COPD model rats impacted the function of bFGF, or some other factor. Therefore, future studies are required for further confirmation.
Acknowledgement
This study was funded by the National Natural Science Foundation of China (Grant No. 81260004).
References
- Hackett TL, Knight DA, Sin DD. Potential role of stem cells in management of COPD. Int J Chron Obstruct Pulmon Dis 2010; 5: 81-88.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176: 532-555.
- Battler A, Scheinowitz M, Bor A, Hasdai D, Vered Z. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J Am Coll Cardiol 1993; 22: 2001-2006.
- Miyataka M, Ishikawa K, Katori R. Basic fibroblast growth factor increased regional myocardial blood flow and limited infarct size of acutely infarcted myocardium in dogs. Angiology 1998; 49: 381-390.
- Kassem M. Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning Stem Cells 2004; 6: 369-374.
- Risbud MV, Albert TJ, Guttapalli A, Vresilovic EJ, Hillibrand AS, Vaccaro AR. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine 2004; 29: 2627-2632.
- Sellke FW, Laham RJ, Edelman ER, Pearlman JD, Simons M. Therapeutic angiogenesis with basic fibroblast growth factor: technique and early results. Ann Thorac Surg 1998; 65: 1540-1544.
- Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol 2004; 10: 3016-3020.
- Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med 2004; 14: 1035-1041.
- Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood 2003; 102: 3483-3493.
- Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976) 2004; 29: 1971-1979.
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 2003; 100: 8407-8411.
- Rojas M, Xu J, Woods CR, Mora AL, Spears W. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005; 33: 145-152.
- Schoeberlein A, Holzgreve W, Dudler L, Hahn S, Surbek DV. Tissue-specific engraftment after in utero transplantation of allogeneic mesenchymal stem cells into sheep fetuses. Am J Obstet Gynecol 2005; 192: 1044-1052.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143-147.
- Ambalavanan N, Novak ZE. Peptide growth factors in tracheal aspirates of mechanically ventilated preterm neonates. Pediatr Res 2003; 53: 240-244.
- Pavlisa G, Pavlisa G, Kusec V, Kolonic SO, Markovic AS. Serum levels of VEGF and bFGF in hypoxic patients with exacerbated COPD. Eur Cytokine Netw 2010; 21: 92-98.
- Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC. Stem cell transplantation: the lung barrier. Transplant Proc 2007; 39: 573-576.
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 2009; 18: 683-692.
- Morino S, Nakamura T, Toba T, Takahashi M, Kushibiki T. Fibroblast growth factor-2 induces recovery of pulmonary blood flow in canine emphysema models. Chest 2005; 128: 920-926.
- Morino S, Toba T, Tao H, Araki M, Shimizu Y, Nakamura T. Fibroblast growth factor-2 promotes recovery of pulmonary function in a canine models of elastase-induced emphysema. Exp Lung Res 2007; 33: 15-26.