Keywords |
| Design, Synthesis, Thieno [2, 3-d] pyrimidines, Anti-breast cancer activity |
Introduction |
| According to WHO population-based data, cancer is a
leading cause of mortality worldwide accounting for
almost 13% of all death [1]. Among all types of cancer,
lung, breast, colorectal, stomach, and prostate cancer is the
underlying cause for the majority of cancer death. Hitherto,
chemotherapy remains one of the therapeutic strategies
adopted worldwide for the management of cancer either
alone or in conjunction with surgery and/or radiotherapy.
Currently in clinical use anticancer agents suffer from a
number of drawbacks correlated to drugs? associated side
effects and/or tumors? multi-drug resistance [2,3]. Hence,
it obviously is still of interest to search for new bioactive
molecules having anticancer activity. Thiophenes
and thienopyrimidines have been reported to possess
interesting biological and pharmacological activities where
several derivatives are used as antibacterial [1-3], antiinflammatory
[4], anticancer [5,6], and antiviral agents [7]. |
| From the chemical and structural point of view, literature
survey showed that sulfonamide [4-8], bearing molecules
play an important role in the anticancer activity. In
addition, various compounds with a heterocyclic backbone
scaffold demonstrated promising anticancer activity. For example, a number of thienopyrimidine derivatives
were claimed to possess interesting anticancer activities
[9,10]. Sulfonamides anticancer activity has been in many
instances attributed to inhibition of carbonic anhydrase
enzymes [4-6]. Carbonic anhydrases (CA, EC 4.2.1.1)
represent a family of Zn based metallo enzymes that
catalyzes the interconversion between carbon dioxide
and bicarbonate with generation of protons. The carbonic
anhydrase isozyme IX (CA IX) is reported to be associated
with tumorogenesis being highly over expressed in hypoxic
tumors and restrictedly expressed in normal tissues [11-14].
CA IX inhibitors have been shown to display promising
anticancer activity in addition to having fewer side effects
compared to other anticancer drugs. Many research
endeavors have reported sulfonamide bearing molecules
as promising anticancer agents acting through inhibition
of carbonic anhydrase IX [11-14]. Most cancer patients are
subjected to chemotherapy for the treatment of advanced
cancers. However, most metastatic solid tumors eventually
remain incurable even by treatment with recent anticancer
drugs. Also, Cancer is a disease of striking significance in
the world today. Although chemotherapy is the mainstay
of cancer therapy, the use of available chemotherapeutic is
often limited mainly due to undesirable side effects and a limited choice of available anticancer drugs [15-17]. This
clearly underlines the urgent need for developing novel
chemotherapeutic agents with more potent anticancer
activities. Many anticancer agents which act as tyrosine
kinase inhibitors comprised the pyrimidine nucleus as
a core moiety. This could be exemplified by different
quinazoline derivatives such as gefitinib (IressaTM) [18]
and tandutinib (MLN518) (phase II clinical trials [19]
(Figure 1). In continuation of our work [20-24], it seemed
of interest to design and synthesize a novel series of
thienopyrimidines bearing biologically active sulfonamide
moieties, analogues to gefitinib (IressaTM) and tandutinib
(MLN518) to evaluate their anti-breast cancer activity. |
Experimental |
| Melting points (oC, uncorrected) were determined in
open capillaries on a Gallenkemp melting point apparatus
(Sanyo Gallenkemp, Southborough, UK). Precoated
silica gel plates (silica gel 0.25 mm, 60 G F 254; Merck,
Germany) were used for thin layer chromatography,
dichloromethane/methanol (9.5:0.5 ml) mixture was
used as a developing solvent system and the spots were
visualized by ultraviolet light and/or iodine. Infrared
spectra were recorded on KBr discs using IR-470
Shimadzu spectrometer (Shimadzu, Tokyo, Japan).
1H-NMR spectra (in DMSO-d6) were recorded on Bruker
Ac-300 ultra-shield NMR spectrometer (Bruker, Flawil,
Switzerland, δ ppm) at 300 MHz, using TMS as internal
standard. Electron impact Mass Spectra were recorded
on a, Shimadzu Gc-Ms-Qp 5000 instruments (Shimadzu,
Tokyo, Japan). Elemental analyses were performed on
Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau,
Germany). All compounds were within ± 0.4% of the
theoretical values. |
Results |
General Procedure for the synthesis of novel
thienopyrimidine derivatives 9- 14. |
| A mixture of ethyl 2-isothiocyanato-4,
5-dimethylthiophene-3- carboxylate 2 (2.41 g, 0.01 mole),
sulfa-drugs (0.012 mole) in dimethylformamide (20 ml)
containing 3 drops of triethylamine was heated under
reflux for 14 h. The reaction mixture was allowed to cool,
filtered off the solid obtained and recrystallized from
dioxane to give compounds 9-14, respectively. |
Synthesis of 4-(5, 6-dimethyl-4-oxo-2-thioxo-1, 2-dihydrothieno[
2, 3-d]pyrimidin-3(4H) yl)benzenesulfonamide(
9). |
| Yield, 86%; m.p. 279.0°C; IR (KBr, cm-1): at 3437, 3425,
3263 (NH, NH2), 3100 (CH arom.), 2978, 2947(CH
aliph.), 1674(C=O), 1338, 1161(SO2), 1234 (C=S).1HNMR
(DMSO-d6) δ: 2.2, 2.3 [2s, 6H, 2CH3], 7.3-8.2 [m,
6H, Ar-H + SO2NH2], 11.2 [s, 1H, NH, exchangeable with
D2O]. 13C-NMR (DMSO-d6): 11.2, 12.4, 115.8, 120.4 (2),
128.8 (2), 130.7, 131.8, 133.9, 134.6, 148.2, 159.7, 180.1.
MS m/z (%): 367 [M+] (20.18), 151 (100). Anal. Calcd.
for C14H13N3O3S3: C, 45.76; H, 3.57; N, 11.44. Found: C,
45.48; H, 3.25; N, 11.12. |
Synthesis of 4-(5, 6-dimethyl-4-oxo-2-thioxo-1,
2-dihydrothieno[2, 3-d]pyrimidin-3(4H)-yl)-N-(thiazol-
2-yl)benzenesulfonamide(10). |
| Yield, 90%; m.p. 205.1°C; IR (KBr, cm-1): 3383, 3375
(NH), 3078 (CH arom.), 2978, 2947(CH aliph.), 1654
(C=O), 1593(C=N), 1388, 1141(SO2), 1238 (C=S). 1HNMR
(DMSO-d6) δ:2.1, 2.2 [2s, 6H, 2CH3], 6.8-8.2 [m,
6H, Ar-H], 8.7 [s, 1H, SO2NH, exchangeable with D2O],
11.1 [s, 1H, NH, exchangeable with D2O]. 13C-NMR (DMSO-d6): 12.2, 13.1, 110.6, 115.8, 119.8 (2), 127.3 (2),
129.6, 130.8, 132.6, 133.2, 134.8, 150.2, 162.1, 169.7,
178.4. MS m/z (%): 450 [M+] (36.53), 93 (100). Anal.
Calcd. for C17H14N4O3S4: C, 45.32; H, 3.13; N, 12.43.
Found: C, 45.68; H, 2.85; N, 11.15. |
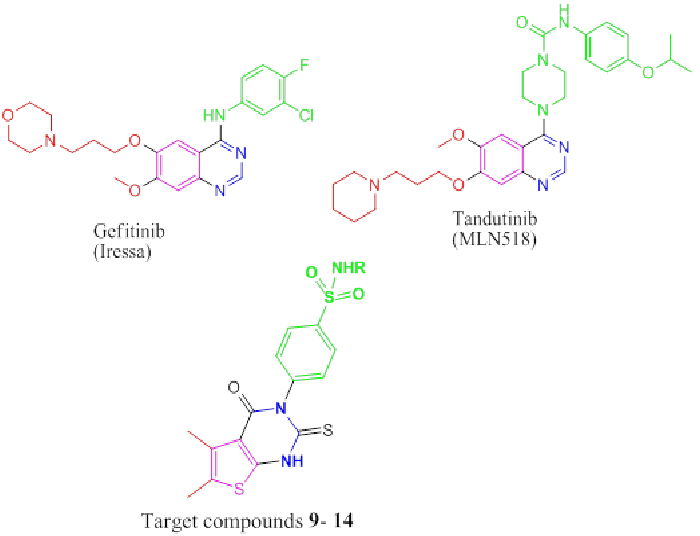 |
| Figure 1: Biologically active (phase II clinical trials) and Target compounds 9-14 |
Synthesis of 4-(5, 6-dimethyl-4-oxo-2-thioxo-1, 2-
dihydrothieno [2, 3-d] pyrimidin-3(4H)-yl)-N-(pyrimidin-
2-yl) benzenesulfonamide(11). |
| Yield, 84%; m.p. 195.3°C; IR (KBr, cm-1): 3425, 3124
(NH), 3109 (CH arom.), 2947, 2924 (CH aliph.), 1685
(C=O), 1577(C=N), 1342, 1161(SO2), 1234 (C=S). 1H
NMR (DMSO-d6) δ:2.2, 2.3 [2s, 6H, 2CH3], 7.1-8.5 [m,
6H, Ar-H], 9.1 [s, 1H, SO2NH, exchangeable with D2O],
11.3 [s, 1H, NH, exchangeable with D2O]. 13C-NMR
(DMSO-d6): 10.8, 12.7, 114.3, 115.6, 120.4 (2), 129.6 (2),
130.3, 131.1, 133.2, 134.0, 151.2, 155.6 (2), 158.0, 167.4.
182.0. MS m/z (%): 445 [M+] (6.23), 156 (100). Anal.
Calcd. for C18H15N5O3S3: C, 48.52; H, 3.39; N, 15.72.
Found: C, 48.26; H, 3.71; N, 15.44. |
Synthesis of 4-(5, 6-dimethyl-4-oxo-2-thioxo-1, 2- dihydrothieno
[2, 3-d] pyrimidin-3(4H)-yl)-N-(4, 6-dimethylpyrimidin-
2-yl)benzenesulfonamide(12). |
| Yield, 88%; m.p. 227.8°C; IR (KBr, cm-1): 3425, 3120
(NH), 3100 (CH arom.), 2974, 2947 (CH aliph.), 1685
(C=O), 1597(C=N), 1381, 1161(SO2), 1230 (C=S).1H
NMR (DMSO-d6) δ:2.1, 2.3 [2s, 6H, 2CH3], 2.4 [s, 6H,
2CH3 pyrimidine], 6.6-8.3 [m, 5H, Ar-H], 8.9 [s, 1H,
SO2NH, exchangeable with D2O], 10.9 [s, 1H, NH,
exchangeable with D2O]. 13C-NMR (DMSO-d6): 10.7,
12.3, 23.5 (2), 111.7, 115.0, 120.6 (2), 128.7 (2), 129.4,
130.6, 132.8, 133.7, 150.0, 159.6, 165.4 (2), 166.7. 181.8.
MS m/z (%): 473 [M+] (2.63), 184 (100). Anal. Calcd.
for C20H19N5O3S3: C, 50.72; H, 4.04; N, 14.79. Found: C,
50.48; H, 3.69; N, 14.48. |
Synthesis of N-(2, 6-dimethoxypyrimidin-4-yl)-4-(5,
6-dimethyl-4-oxo-2-thioxo-1, 2- dihydrothieno [2, 3-d]
pyrimidin-3(4H)-yl)benzenesulfonamide(13). |
| Yield, 83%; m.p. 296.4°C; IR (KBr, cm-1): 3340, 3186
(NH), 3055 (CH arom.), 2941, 2836 (CH aliph.), 1703
(C=O), 1618 (C=N), 1382, 1153 (SO2), 1244 (C=S).
1H-NMR (DMSO-d6) δ:2.2, 2.3 [2s, 6H, 2CH3], 3.8 [s,
6H, 2OCH3], 5.8 [s, 1H, CH pyrimidine], 7.0-8.0 [m,
4H, Ar-H], 8.9 [s, 1H, SO2NH, exchangeable with D2O],
10.6 [s, 1H, NH, exchangeable with D2O]. 13C-NMR
(DMSO-d6): 11.2, 12.4, 55.2, 55.6, 81.7, 115.6, 120.2 (2),
127.0 (2), 129.6, 130.8, 132.9, 133.4, 151.0, 155.5, 158.7,
166.2, 169.8, 181.6. MS m/z (%): 505 [M+] (12.18), 153
(100). Anal. Calcd. for C20H19N5O5S3: C, 47.51; H, 3.79;
N, 13.85. Found: C, 47.25; H, 3.99; N, 14.17. |
Synthesis of N-(5, 6-dimethoxypyrimidin-4-yl)-4-(5,
6-dimethyl-4-oxo-2-thioxo-1, 2- dihydrothieno [2, 3-d]
pyrimidin-3(4H)-yl) benzenesulfonamide(14). |
| Yield, 79%; m.p. 130.6°C; IR (KBr, cm-1): 3110 (NH), 3012
(CH arom.), 2981, 2945, 2868(CH aliph.), 1656(C=O), 1583(C=N), 1377, 1163(SO2), 1240 (C=S). 1H-NMR
(DMSO-d6) δ:2.1, 2.2 [2s, 6H, 2CH3], 3.8, 3.9 [2s, 6H,
2OCH3], 7.1-8.5 [m, 5H, Ar-H], 9.1 [s, 1H, SO2NH,
exchangeable with D2O], 11.0 [s, 1H, NH, exchangeable
with D2O]. 13C-NMR (DMSO-d6): 10.6, 12.4, 54.0,
55.6, 115.4, 119.7 (2), 128.3 (2), 129.7, 129.9, 131.0,
132.8, 133.4, 150.1, 151.8, 152.6, 157.7, 162.0, 182.6.
MS m/z (%): 505 [M+] (7.56), 138 (100). Anal. Calcd.
for C20H19N5O5S3: C, 47.51; H, 3.79; N, 13.85. Found: C,
47.84; H, 3.54; N, 13.51.(Scheme 1) |
In vitro Anticancer Activity |
| The cytotoxic activity was measured in vitro for the newly
synthesized compounds using the SulfoRhodamine-B
stain (SRB) assay using the Skehan et al. [25]. The in vitro
anticancer screening was done at the Pharmacology Unit,
the National Cancer Institute, Cairo University. Cells were
plated in 96-multiwall microliter plate (104-cells/well)
for 24h before treatment with the compound(s) to allow
attachment of cell to the wall of the plate. Test compounds
were dissolved in DMSO and diluted with saline to the
appropriate volume. Different concentrations of the
compound under test (10, 25, 50 and 100 μM) were added
to the cell monolayer. Triplicate wells were prepared for
each individual dose. Monolayer cells were incubated
with the compound (s) for 48 h at 37°C and in atmosphere
of 5% CO2. After 48 h, cells were fixed, washed, and
stained for 30 min with 0.4% (W/V) with SRB dissolved
in 1% acetic acid. Excess unbound dye was removed by
four washes with 1% acetic acid and attached stain was
recovered with Tris-EDTA buffer. Color intensity was
measured in an enzyme- linked immunosorbent assay
ELISA reader. The relation between surviving fraction
and drug concentration is plotted to get the survival curve
for breast tumor cell line after the specified time [25]. The
molar concentration required for 50% inhibition of cell
viability (IC50) was calculated and the results are given
in (Table 1).The relationship between surviving fraction
and drug concentration was plotted to obtain the survival
curve of breast cancer cell line (MCF7). The response
parameter calculated was IC50 value, which corresponds
to the concentration required for 50% inhibition of cell
viability. |
In vitro Anti-Breast Cancer Activity |
| The newly synthesized compounds were evaluated for their
in vitro anticancer activity against human breast cancer
cell line, MCF7. Doxorubicin, which is one of the most
effective anticancer agents, was used as the reference drug
in this study. The relationship between surviving fraction
and drug concentration was plotted to obtain the survival
curve of breast cancer cell line (MCF7). The response
parameter calculated was the IC50 value, which corresponds
to the concentration required for 50% inhibition of cell
viability. Table 1 shows the in vitro cytotoxic activity of
the newly synthesized compounds. Most of the tested compounds exhibited significant activity compared to the
Doxorubicin as reference drug. From the results of Table 1,
it was found that thienopyrimidine containing biologically
active sulfa-doxine at 3-position with thione moiety at
2-position 14, sulfa-dimethoxazine at 3-position, with
thione moiety at 2-position 13, sulfanilamide at 3-position
with thione moiety at 2-position 9, and sulfa-merazine at
3-position with thione moiety at 2-position 12 with IC50
values (22.12, 22.52, 27.83, and 29.22 μM) exhibited more
potent anti-breast cancer activity than the reference drug
with IC50 value (30.40 μM). Farther, thienopyrimidine
bearing the biologically active sulfa-thiazole at 3-position
with thione moiety at 2-position 10 and sulfa-diazine
at 3-position with thione moiety at 2-position 11 with
IC50 values (34.64, 37.78 μm) are nearly as active as
Doxorubicin as positive control. |
Discussion |
| The compounds were designed in the aim of exploring
their anti-breast cancer activity. The sequence of reaction
followed in the synthesis of the target compounds is
illustrated in (Scheme 1). As a part of a program aimed at
the synthesis of novel thieno [2, 3-d]pyrimidine derivatives
having the biologically active sulfonamide moieties
9-14, namely sulfanilamide 9, sulfa- thiazole10, sulfadiazine11,
sulfa- merazine12, sulfa- dimethoxazine13
and sulfa- doxine14, which could be useful for biological
screening, we have investigated the possible utility of
2-isothiocyanatothiophene 2 [26] to react with sulfa- drugs
in dimethylformamide in the presence of trimethylamine
as catalyst to give novel thienopyrimidine derivatives 9-
14 in high yield (Scheme 1). Thus, treatment of 2 with
sulfa-drugs in refluxing dimethylformamide in presence of triethylamine as catalyst furnished the corresponding
sulfonamide derivatives 9-14, through the formation of
intermidiates 3-8. The structures of the later products
were assigned on the basis of their analytical and spectral
data. The IR spectra of the reaction products showed in
each case three absorption bands corresponding to NH
functions in the region 3425-3110 cm-1, in addition to a
carbonyl absorption band in the region 1703-1654 cm-
1,absorption bands assigned to C=S function in the region
1244- 1230 cm-1, absorption bands due to SO2 functions
in the region 1388-1141 cm-1. IR spectrum of compound
9 revealed the absence of N=C=S group and presence of
characteristic bands at 3437, 3425, 3263 cm-1 (NH, NH2),
3100 cm-1 (CH arom.), 2978, 2947 cm-1 (CH aliph.), 1674
cm-1 (C=O), 1338, 1161 cm-1 (SO2), 1234 cm-1 (C=S).
1H-NMR spectrum of 9 in (DMSO-d6) revealed singlet at
11.2 ppm assigned to NH group, 13C-NMR spectrum of
compound 9 exhibited singlet at 180.1 ppm attributed to
C=S group. Compound 10 was established on the basis
of elemental analysis and spectral data. IR spectrum of
compound 10 showed the absence of N=C=S group and
presence of characteristic bands at 3383, 3375 cm-1 (NH),
3078 cm-1 (CH arom.), 2978, 2947 cm-1 (CH aliph.), 1654
cm-1 (C=O), 1593 cm-1 (C=N), 1388, 1141 cm-1 (SO2),
1238 cm-1 (C=S). 1H-NMR spectrum of 10 in (DMSO-d6)
revealed signals at 8.7, 11.1 ppm due to SO2NH and NH
groups. 13C-NMR spectrum of 10 showed singlet at 178.4
ppm for C=S group. Compound 11 was proved on the
basis of elemental analysis and spectral data. IR spectrum
of compound 11 exhibited the absence of N=C=S group
and presence of characteristic bands at 3425, 3124 cm-1
(NH), 3109 cm-1 (CH arom.), 2947, 2924 cm-1 (CH
aliph.), 1685 cm-1 (C=O), 1577 cm-1 (C=N), 1342, 1161
cm-1 (SO2), 1234 cm-1 (C=S). 1H-NMR spectrum of 11 in
(DMSO-d6) showed signals at 9.1, 11.3 ppm due to SO2NH
and NH groups. 13C-NMR spectrum of 11 exhibited
singlet at 182.0 ppm assigned C=S group. Compound 12
was established on the basis of elemental analysis and
spectral data. IR spectrum of compound 12 showed the
absence of N=C=S group and presence of characteristic
bands at 3425, 3120 cm-1 (NH), 3100 cm-1 (CH arom.),
2974, 2947 cm-1 (CH aliph.), 1685 cm-1 (C=O), 1597
cm-1 (C=N), 1381, 1161 cm-11 (SO2), 1230 cm-1 (C=S).
1H-NMR spectrum of 12 in (DMSO-d6) revealed singlet
at 2.4 ppm attributed to CH3 group for pyrimidine ring.
13C-NMR spectrum of 12 revealed singlet at 181.8 ppm
according to C=S group. Compound 13 was elucidated
on the basis of elemental analysis and spectral data. IR
spectrum of compound 13 revealed the absence of N=C=S
group and presence of characteristic bands at 3340, 3186
cm-1 (NH), 3055 cm-1 (CH arom.), 2941, 2836 cm-1 (CH
aliph.), 1703 cm-1 (C=O), 1618 cm-1 (C=N), 1382, 1153
cm-1 (SO2), 1244 cm-1 (C=S). 1H-NMR spectrum of 13 in
(DMSO-d6) showed singlet at 3.8 ppm assigned to 2OCH3
groups. 13C-NMR spectrum of 13 showed signals at 55.2,
55.6 ppm attributed to 2OCH3 groups. Compound 14 was
proved on the basis of elemental analysis and spectral data. IR spectrum of compound 14 exhibited the absence of
N=C=S group and presence of characteristic bands at 3110
cm-1 (NH), 3012 cm-1 (CH arom.), 2981, 2945, 2868 cm-1
(CH aliph.), 1656 cm-1 (C=O), 1583 cm-1 (C=N), 1377,
1163 cm-1 (SO2), 1240 cm-1 (C=S). 1H-NMR spectrum of
14 in (DMSO-d6) showed signals at 3.8, 3.9 ppm assigned
to 2OCH3 groups, while its 13C-NMR spectrum exhibited
singlet at 182.6 ppm according to C=S group. |
Conclusion |
| The objective of the present study was to synthesize
and investigate the anti-breast cancer activity of some
novel thieno [2,3-d] pyrimidine derivatives carrying
the biologically active benzenesulfonamide moieties at
3-position and thione moiety at 2-position. Compounds
14 bearing sulfa-doxine at 3-position, thione moiety at
2-position, 13 having sulfa-dimethoxazine, 9 carrying the
corresponding sulfanilamide and 12 incorporating sulfamerazine
with IC50 values (22.12, 22.52, 27.83, 29.22 μM)
were found the most active compounds compared with
Doxorubicin as reference drug, while compounds 10 and
11 with IC50 values (34.64, 37.78 μM) are nearly as active
as Doxorubicin as positive control. |
Acknowledgement |
| The authors would like to extend their sincere appreciation
to the Deanship of Scientific Research at King Saud
University for its funding of this research through the
Research Group Project no. RGP-VPP-302. |
References |
- WHO, Cancer, World Health Organization 2006.
- Matsuo H, Wakasugi M, Takanaga H, Ohtani H, Naito M, Tsuruo T, Sawada Y, Possibility of the reversal of multidrug resistance and the avoidance of side effects by liposomes modified with MRK-16, a monoclonal antibody to P-glycoprotein. J Control Release2001; 77: 77-86.
- Fahad-Ullah M. Cancer multidrug resistance (MDR): A major impedimentto effective chemotherapy. Asian Pac J Cancer Preven 2008; 9: 1-6.
- Marques SM, Enyedy EA, Supuran CT, Krupenko NI, Krupenko SA, Santos MA. Pteridine?sulfonamide conjugates as dual inhibitors of carbonic anhydrases and dihydrofolate reductase with potential antitumor activity. Bioorg. Med. Chem2010; 18: 5081-5089.
- Ghorab MM, Ragab FA, Heiba HI, Youssef HA, El-Gazzar MG. Synthesis of novel pyrazole and pyrimidine derivatives bearing sulfonamide moiety as antitumor and radiosensitizing agents. Med Chem Res 2012; 21: 1376-1383.
- Ghorab MM, Ragab FA, Heiba HI, Arafa RK, El-Hossary EM. In vitro anticancer screening and radiosensitizing evaluation of some new quinolines and pyrimido [4,5-b]quinolines bearing a sulfonamide moiety.Eur J Med Chem 2010; 45: 3677-3684.
- Abbate F, CasiniA, Owa T, Scozzafava A, Supuran CT.Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. Bioorg Med ChemLett 2004; 14: 217-223.
- Stephens CE, Felder TM, Sowell JW, Andrei G, Balzarini J, Snoeck R, Clercq ED. Synthesis and antiviral/antitumor evaluation of 2-amino- and 2-carboxamido-3-arylsulfonylthiophenes and related compounds as a new class of diarylsulfones. Bioorg Med Chem 2001; 9: 1123-1132.
- Wahid MB, Tamer KK, Fakhry AE, Ahmed RH, Effcient and expeditions synthesis of pyrano-pyrimidines, multi-substituted ?- pyrans, and their antioxidant activity. J Heterocyclic Chem 2014; 51: 106 ? 115.
- Amr AE, Mohamed AM, Mohamed SF, Abdel-Hafez NA, Hammam AG, Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg Med Chem 2006; 14: 5481?5488.
- Supuran CT. Inhibition of carbonic anhydrase IX as a novel anticancer mechanism. World J ClinOncol 2012; 3: 98?103.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008; 7: 168-181.
- Said HM, Supuran CT, Hageman C, Staab A, Polat B, Katzer A, Scozzafava A, Anacker J, Flentje M, Vordermark D. Modulation of carbonic anhydrase 9 (CA9) in human brain cancer. Curr Pharm Des 2010; 16: 3288-3299.
- Supuran CT. Carbonic anhydrases as grug targets executive. Curr Pharm Des 2008; 14: 601-602.
- Gibbs JB. Mechanism-based target identification and drug discovery in cancer research. Science 2000; 287:1969-1971.
- Unger C. New therapeutic approaches in cancer treatment. Drug Future 1997; 22: 1337-1345.
- Solomon VR, Hu C, Lee H. Hybrid pharmacophore design and synthesis of isatin?benzothiazole analogs for their anti-breast cancer activity. Bioorg Med Chem 2009; 17: 7585-7592.
- Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. Cancer Res 2002; 62: 5749-5754.
- DeAngelo DJ, Stone RM, Heany ML, Nimer SD, Paquette RL, Klisovic RB, Caligiuri MA, Cooper MR, Leverf J, Karol MD, Sheng S, Holford N, Curtin PT, Druker BJ, Heinrich C. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelo-dysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics.Blood2006;108: 3674-3681.
- Ghorab MM, Alsaid MS, Ghabour HA, Fun HK. Synthesis, crystal structure and antitumor Activity of novel 2-cyano-N- (quinolin-3-yl) acetamide. Asian Journal of Chemistry 2014; 26: 7389-7392.
- Ghorab MM, Alsaid MS, Nissan YM. Dapson in heterocyclic chemistry, part V: Synthesis, molecular docking and anticancer activity of some novel sulfonylbis-compounds carrying biologically active dihydropyridine, dihydroisoquinoline, 1,3-dithiolan, 1,3-dithian, acrylamide, pyrazole, pyrazolopyrimidine and benzochromenemoieties. Chem Pharm Bull 2012; 60: 1019-1028.
- Ghorab MM, Ragab FA, Heiba HI, Nissan YM, Ghorab WM. Novel brominated quinoline and pyrimidoquinoline derivatives as potential cytotoxic agents with synergistic effects of gamma-radiation.Arch Pharm Res 2012; 8: 1335-1346.
- Ghorab MM, Shaaban MA, Refaat HM, Heiba HI, Ibrahim SS. Anticancer and radiosensitizing evaluation of some new pyranothiazole-Schiff bases bearing the biologically active sulfonamide moiety. Eur J Med Chem 2012; 53: 403-407.
- Al-Dosari MS, Ghorab MM, Alsaid MS, Nissan YM, Ahmed AB. Synthesis and anticancer activity of some novel trifluoromethylquinolines carrying a biologically active benzenesulfonamide moiety. Eur J Med Chem 2013; 69: 373-383.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer- drug screening. J Natl Cancer Inst 1990; 82: 1107-1112.
- Heba HI, Ragab FA, Noaman E, Ghorab MM, Galal M. Synthesis of some novel sulfur containing triazolothienopyrimidines and biscompounds as possible antitumor and radioprotective agents. Arzneimittelforschung 2006; 56: 593-599.
|
