ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
A surveillance study on the prevalence and antimicrobial resistance pattern among different groups of bacteria isolated from Western province of Saudi Arabia
1King Fahd Medical Research Center, King Abdulaziz University, P. O. Box 80216, Jeddah 21589, Saudi Arabia
2Department of Medical Microbiology and Parasitology, Faculty of Medicine, King Abdulaziz University, P.O. Box 80205, Jeddah 21589, Saudi Arabia
3Clinical and Molecular Microbiology Laboratory, King Abdulaziz University Hospital, P.O. Box 80205, Jeddah 21589, Saudi Arabia
- *Corresponding Author:
- Absarul Haque
King Fahd Medical Research Center
King Abdulaziz University, Saudi Arabia
E-mail: absar99@gmail.com
Accepted date: July 06, 2016
Objective: The epidemiological data from a given location are useful for antimicrobial therapy. This study was carried out to find new insight regarding the prevalence of drug resistance among pathogenic bacteria from Western region of Saudi Arabia.
Methods: The antimicrobial resistance profiles were investigated in bacteria from various specimens like blood, skin, wound, stool, urine, and respiratory tract. In vitro susceptibilities were determined by VITEK 2 system using AST-GN69 and AST-GP67 cards for Gram-negative bacteria and Gram-positive respectively.
Results: Among 877 E. coli isolates from urine, 48.6% were found resistant to SXT, 49.3% of 341 Klebsiella isolates were resistant to AMP. Eighty (59.3%) of Klebsiella isolates from respiratory secretions were resistant to AMP and Pipracillin. Of 244 Acinetobacter baumannii from respiratory sources 20.1% were resistant to SXT. Twenty seven (56.3%) E. coli and 31.1% Klebsiella from respiratory sources were positive for Extended Spectrum Beta Lactamases (ESBLs). Methicillinresistance was observed in 50.3% S. aureus from miscellaneous cultures whereas 35.9% were methicillin resistant among respiratory isolates.
Conclusion: The study provides the information regarding the drug resistance patterns in Escherichia coli, Klebsiella and other bacterial pathogens from Western Region of Saudi Arabia. The study is significant in terms of prevalence of ESBL positive and methicillin resistant S. aureus (MRSA) isolates in the western region of Saudi Arabia.
Introduction
The growing incidence of antimicrobial resistance is the key concern globally and it is considered as one of the main hurdle in the treatment of patients suffering from bacterial infections. It is estimated that around 1.3 to 2 fold rise in mortality caused by antimicrobial resistant bacteria compared with susceptible infections [1,2]. Antimicrobial resistance is described as a condition in which microorganism such as bacteria escape the stress of the antibiotic exposure [3]. The indiscriminate use of antibiotics by clinicians has made a great cause in the emergence of Multi Drug Resistance (MDR) bacteria, which occurs when a bacterium carries multiple resistance genes simultaneously. The appearance of MDR bacteria in recent years has also resulted in increased morbidity and mortality due to infectious diseases [4]. The pandemic spread of MDR bacteria has posed a great amount of burden to the society as many more healthcare systems and new setup are required to monitor and control the infectious diseases.
The bacterial infections are mostly treated by β-lactam antimicrobial drugs all over the world. The β-lactamases and Extended Spectrum β-Lactamases (ESBLs) are the bacterial enzymes that hydrolyze β-lactam ring of the drugs, is the primary mechanism, which provides resistance to a bacterium against β-lactam antibiotics [5]. The morbidity and mortality associated with the emergence of methicillin-resistant S. aureus (MRSA) has increased drastically around the world, and hence, the management of such infections imposes high and significant amount of burden on healthcare resources [6,7]. MRSA isolates have been reported to become resistant to several other antimicrobials simultaneously [8]. Notably, there are only few studies available regarding the prevalence of MRSA and ESBL producing strains in Saudi Arabia [9-12].
The epidemiological data and antibiograms from a given region are useful to optimize empirical antimicrobial therapy. The epidemiological studies related to the problem of drug resistance in bacteria isolated from Western region of Saudi Arabia have rarely been addressed in a comprehensive way. Antimicrobial susceptibility pattern of pathogens can vary markedly between hospitals and different units within the same hospital. It is therefore, there is utmost need to determine the prevalence and antimicrobial susceptibility patterns of the most common infecting bacterial species in order to adopt appropriate treatment modalities in the light of the new findings based on antimicrobial susceptibility pattern among the particular community or region. The current study was carried out to find some new insight regarding the prevalence and drug susceptibility pattern among different groups of bacteria recovered from Western region of Saudi Arabia. The prevalence of MRSA and ESBLs production were also analyzed in these resistant bacterial isolates.
Materials and Methods
Samples collection
Bacterial isolates both Gram-positive and Gram-negative, were collected from three different types of specimens like urinary and respiratory sources and miscellaneous specimens, which include skin wounds, blood, stool, and catheters. All samples were collected from patients visiting King Abdulaziz University Hospital, Jeddah, Saudi Arabia using standard laboratory procedures between September 2013 and May 2014. The study was approved by the Research Ethics Committee of the Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
Identification of bacterial isolates
The specimens were inoculated on sheep blood and mannitol salt agar plates (Saudi Prepared Media Laboratory, Riyadh) using sterile disposable loops. Plates were incubated at 35-37ºC for 24-48 h. Preliminary identification of isolates was performed by conventional methods like colony morphology, Gram’s staining, and culture characteristics on media. Further identification of bacterial isolates up to species level was performed using VITEK 2 technology system using ASTGN69 and AST-GP67 cards (BioMérieux, France) for Gramnegative and Gram-positive isolates respectively according to the manufacturer´s instructions.
Antimicrobial susceptibility tests
The isolated bacteria were screened for their antimicrobial susceptibility patterns using the AST-GN69 and AST-GP67 cards for Gram-negative and Gram-positive isolates respectively on VITEK 2 technology system (BioMérieux, France) according to the manufacturer’s instruction. The detection of MRSA and ESBL producers were determined according to the criteria as described in information leaflets of the above mentioned cards. Following antimicrobials were tested for resistance patterns: Amikacin (AK); Ampicillin (AMP); Amoxicillin/Clavulanic acid (AMC); Cefazolin (CFZ); Cefuroxime (CXM); Ceftriaxone (CRO); Cefotaxime (CTX); Ceftazidime (CAZ); Cefepime (FEP); Ciprofloxacin (CIP); Clindamycin (DA); Oxacillin (OX); Erythromycin (E); Gentamicin (GN); Imipenem (IMP); Meropenem (MEM); Nitrofurantoin (NIT); Penicillin (P); Pipracillin (PRL); Piperacillin/Tazobactam (TZP); Tigecycline (TG); Trimethoprim/Sulfamethoxazole (SXT); Vancomycin (VA); Streptomycin (S); Levofloxacin (LEV). E. coli ATCC 25922, Enterococci ATCC29212, S. aureus ATCC 25923, Pseudomonas ATCC 27853, and Streptococci agalactiae ATCC 12386 were used as quality control strains. The data of antimicrobial resistance pattern are presented in terms of number of isolates and their percentages in parentheses.
Results
Incidence of antimicrobial resistance
The most common bacterial pathogen isolated from urine was E. coli totaling to 877 isolates. Out of these 877 E. coli isolates, 48.6% were resistant to SXT followed by PRL (46.5%). Among these E. coli isolates, 39.1% were found resistant to AMP (Table 1). Fewer numbers of E. coli were found resistant to CXM, CTX, CAZ, FEP, and TZP. Moreover, none of the E. coli isolate was resistant to CFZ. Klebsiella was the second most prevalent bacteria encountered among the isolates from urinary sources. Of 341 Klebsiella isolates, 49.3% were found exhibiting resistance toward AMP followed by resistance to PRL (46.0%). Klebsiella is known to be intrinsically resistant to AMP. Further, 28.2% Klebsiella isolates were also resistant to SXT. S. aureus isolates were mainly resistant to OX (46.9%) whereas Proteus mirabilis were mostly resistant to NIT (44.3%). The other pathogens from urine like Enterobacter, A. baumannii, Enterococci and Pseudomonas were mainly sensitive to the antimicrobials tested. Among isolates from respiratory sources, the most frequently encountered pathogen was Pseudomonas (n=300) out which 19.7% were resistant to PRL followed by TZP (18.3%). CAZ was found not inhibitory to 14.0% whereas MEM was not inhibitory to 13.7% Pseudomonas isolates (Table 1). A total of 49 isolates (20.1%) of A. baumannii from respiratory sources were found exhibiting resistance to SXT followed by CIP (19.7%), MEM (17.6%) and CAZ (16.4%). Eighty (59.3%) Klebsiella isolates were resistant to AMP and PRL each. The E. coli isolates were 48 out of which 54.2% was resistant to SXT. Among respiratory S. aureus isolates, 29.8% were resistant to OX, 23.0% were resistant to E and 20.2% were resistant to DA. The antimicrobials like CXM, CRO, CTX, FEP, and IMP were found effective against most of the bacterial isolates.
| Bacteria | Source | Antibacterial resistance (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | AMC | CXM | CIP | GN | PRL | TZP | SXT | CAZ | IMP | NIT | MEM | FEP | VA | ||
| E. coli | U (877) | 343 (39.1) | 54 (6.2) | 58 (6.6) 6 (12.5) | 247 (28.2) | 133 (15.2) | 408 (46.5) | 67 (7.6) | 426 (48.6) | 24 (2.7) | - | - | - | - | - |
| R (48) | 19 (39.6) | 2 (4.2) | 24 (8.9) | 16 (33.3) | 14 (29.2) | 20 (41.7) | 4 (8.3) | 26 (54.2) | 8 (16.7) | - | - | - | - | - | |
| M (269) | 81 (30.1) | 20 (7.4) | 88 (7.4) | 112 (41.6) | 46 (17.1) | 101 (37.5) | 16 (5.9) | 140 (52.0) | 9 (3.3) | - | - | - | - | - | |
| Total (1194) | 443 (37.1) | 76 (6.4) | - | 375 (31.4) | 193 (16.2) | 529 (44.3) | 87 (7.3) | 592 (49.6) | 41 (3.4) | - | - | - | - | - | |
| Klebsiella | U (341) | 168 (49.3) | 20 (5.9) | 20 (5.9) | 45 (13.2) | 38 (11.1) | 157 (46.0) | 29 (8.5) | 96 (28.2) | 6 (1.8) | - | 81 (23.8) | - | - | - |
| R (135) | 80 (59.3) | 6 (4.4) | 7 (5.2) | 14 (10.4) | 15 (11.1) | 80 (59.3) | 10 (7.4) | 39 (28.9) | 7 (5.2) | - | - | - | - | - | |
| M (204) | 72 (35.3) | 17 (8.3) | 14 (6.9) | 60 (29.4) | 54 (26.5) | 83 (40.8) | 33 ( 16.2) | 84 (41.2) | 5 (2.5) | - | - | - | - | - | |
| Total (680) | 320 (47.1) | 43 (6.3) | 41 (6.0) | 119 (17.5) | 107 (15.7) | 320 (47.1) | 72 (15.6) | 219 (32.2) | 18 (2.6) | - | 81 (11.9) | - | - | - | |
| Enterobacter | U (39) | 4 (10.3) | 9 (23.1) | - | 4 (10.3) | 5 (12.8) | 7 (17.9) | 5 (12.8) | 9 (23.1) | 5 (12.8) | - | 7 (17.9) | - | - | - |
| R (36) | 13 (36.1) | 7 (19.4) | - | 1 (2.8) | – | 8 (22.2) | 6 (16.7) | 7 (19.4) | 5 (13.9) | - | - | - | - | - | |
| M (66) | 10 (15.2) | 8 (12.1) | - | 5 (7.6) | 6 (9.1) | 5 (7.6) | 5 (7.6) | 13 (19.7) | 4 (6.1) | - | 1 (1.5) | - | - | - | |
| Total (141) | 27 (19.1) | 24 (17.0) | - | 10 (7.1) | 11 (7.8) | 20 (14.2) | 16 (11.4) | 29 (20.6) | 14 (9.9) | - | 8 (5.7) | - | - | - | |
| A. Baumannii | U (93) | 10 (10.8) | - | - | 8 (8.6) | 4 (4.3) | - | 7 (7.5) | 10 (10.8) | 8 (8.6) | 3 (3.2) | 20 (21.5) | 11 (11.8) | 4 (4.3) | - |
| R (244) | 33 (13.5) | - | - | 48 (19.7) | 34 (13.9) | - | 23 (9.4) | 49 (20.1) | 40 (16.4) | 19 (7.8) | - | 43 (17.6) | 28 (11.5) | - | |
| M (190) | 8 (4.2) | - | - | 58 (30.5) | 28 (14.7) | - | 48 (25.3) | 41 (21.6) | 52 (27.4) | 26 (13.7) | - | 42 (22.1) | 50 (26.3) | - | |
| Total (527) | 27 (5.1) | - | - | 114 (21.6) | 66 (12.5) | - | 78 (14.8) | 100 (18.9) | 100 (18.9) | 48 (9.1) | 20 (3.8) | 96 (18.2) | 82 (15.6) | - | |
| P. mirabilis | U (61) | 18 (29.5) | 4 (6.6) | - | 11 (18.0) | 6 (9.8) | 13 (21.3) | - | 27 (44.3) | - | - | 25 (40.9) | - | - | - |
| M (114) | 27 (23.7) | 1 (0.9) | - | 26 (22.8) | 17 (14.9) | 26 (22.8) | - | 50 (43.9) | - | - | 5 (4.4) | - | - | - | |
| Total (175) | 45 (25.7) | 5 (2.9) | - | 37 (21.1) | 23 (13.1) | 39 (22.3) | - | 77 (44.0) | - | - | 30 (17.1) | - | - | - | |
| Enterococci | U ( (270) | 40 (14.8) | - | - | - | 31 (11.5) | - | - | 28 (10.4) | - | 12 (4.4) | - | - | - | 13 (4.8) |
| M (122) | 21 (17.2) | - | - | - | 30 (24.6) | - | - | 13 (10.7) | - | 5 (4.1) | - | - | - | 16 (13.1) | |
| Total (392) | 61 (15.6) | - | - | - | 61 (15.6) | - | - | 41 (10.5) | - | 17 (4.3) | - | - | - | 29 (7.4) | |
| Pseudomonas | U (182) | - | - | - | 14 (7.6) | 13 (7.1) | 20 (10.9) | 13 (7.1) | 8 (4.4) | 12 (6.6) | - | 17 (9.3) | 4 (4.2) | 7 (3.8) | - |
| R (300) | - | - | - | 21 (7.0) | 23 (7.7) | 59 (19.7) | 55 (18.3) | 6 (2.0) | 42 (14.0) | - | – | 41 (13.7) | 20 (6.7) | - | |
| M (289) | - | - | - | 32 (11.1) | 26 (8.9) | 47 (16.3) | 45 (15.6) | 12 (4.2) | 44 (15.2) | - | – | 23 (7.9) | 27 (9.3) | - | |
| Total (771) | - | - | - | 67 (8.7) | 62 (8.0) | 126 (16.3) | 113 (14.7) | 26 (3.4) | 98 (12.7) | - | 17 (2.2) | 68 (8.8) | 54 (7.0) | - | |
| S. aureus | - | GN | SXT | DA | OX | E | - | - | - | - | - | - | - | - | - |
| U (64) | 4 (6.3) | 12 (18.8) | 7 (10.9) | 30 (46.9) | 8 (12.5) | - | - | - | - | - | - | - | - | - | |
| R (178) | 12 (6.7) | – | 36 (20.2) | 53 (29.8) | 41 (23.0) | - | - | - | - | - | - | - | - | - | |
| M (314) | 9 (2.9) | 63 (20.1) | 68 (21.7) | 125 (39.8) | 83 (26.4) | - | - | - | - | - | - | - | - | - | |
| Total (556) | 25 (4.5) | 75 (13.5) | 111 (19.9) | 208 (37.4) | 132 (23.7) | - | - | - | - | - | - | - | - | - | |
| U: Urine; R: Respiratory; M: Miscellaneous; percentage of resistant bacteria is shown in parenthesis. The data of bacterial resistance against those antimicrobials which were most inhibitory are not shown in the Table like CRO, CTX, CFZ, S, P, LEV AK, and TG | |||||||||||||||
Table 1. Antimicrobial resistance profile of different bacteria isolated from various sources.
The patterns of antimicrobial resistance of bacterial isolates from miscellaneous sources like wound, blood, skin and stool are also presented in Table 1. SXT was found most tolerated by E. coli isolates where 52.0% were resistant to it. E. coli isolates were also found resistant to CIP (41.6%) followed by PRL and AMP. Eighty four (41.2%) out of total 204 Klebsiella isolates among miscellaneous sources group were resistant to SXT. AMP was resisted by 35.3% isolates of Klebsiella. Among S. aureus isolates from miscellaneous sources, 39.8% were found resistant to OX followed by erythromycin (26.4%), DA (21.7%), and SXT (20.1%). Among all these isolates, most were sensitive toward AMC, CXM, CRO, CTX, and IMP. The resistance profile of all the isolates from three different groups, namely respiratory, urine and miscellaneous sources are presented in Table 1. Out of total 1194 E. coli, 49.6% were resistant to SXT followed by PRL (44.3%). Among 680 Klebsiella isolates, 47.1% were found showing resistance to AMP and PRL each. Among A. baumannii isolates, 21.6% were found resistant to CIP whereas 37.4% out of total 556 S. aureus isolates were resistant to OX. The antimicrobials like CFZ, CXM, CRO, CTX, MEM and IMP were found inhibitory to most of the bacterial isolates.
Analysis of resistance patterns
Analyzing the pattern of antimicrobials resistance in the isolated bacterial species revealed that most of the isolates were resistant to one or more antimicrobials simultaneously (Table 2). In respiratory isolates, 35.7% A. baumannii were sensitive to all antimicrobials, 15.9%, 10.7%, 8.2% and 5.3% were resistant to one, two, three and four antimicrobials respectively. On the other hand, majority (58.6%) of Pseudomonas isolates were found sensitive to all antimicrobials tested and only 5.3% were resistant to more than five antimicrobials simultaneously. Among isolates of urinary sources, 23.2% E. coli were sensitive to all antimicrobials, 11.6% were found exhibiting resistance to one antimicrobial only and 11.6% isolates were found simultaneously resistant to more than five antimicrobials at a time. Similar to Pseudomonas isolated from respiratory sources, Pseudomonas isolates of urine and miscellaneous sources were mostly sensitive to the antimicrobials tested.
| Source | Bacteria | Bacteria resistant to number of antimicrobials (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | >5 | ||
| Respiratory isolates | E. coli (48) | 11 (22.9) | 8 (16.7) | 8 (16.7) | 7 (14.6) | 2 (4.2) | 4 (8.3) | 8 (16.7) |
| Klebsiella (135) | 27 (20.0) | 17 (12.6) | 52 (38.5) | 12 (8.9) | 14 (10.4) | 8 (5.9) | 5 (3.7) | |
| Enterobacter (36) | 16 (44.4) | 5 (13.9) | 7 (19.4) | 2 (5.6) | 2 (5.6) | 3 (8.3) | 1 (2.8) | |
| A. baumannii (244) | 87 (35.7) | 39 (15.9) | 26 (10.7) | 20 (8.2) | 13 (5.3) | 15 (6.1) | 44 (18.0) | |
| Pseudomonas (300) | 176 (58.6) | 30 (10.0) | 28 (9.3) | 20 (6.7) | 18 (6.0) | 12 (4.0) | 16 (5.3) | |
| S. aureus (178) | 93 (52.2) | 25 (10.0) | 17 (9.6) | 16 (8.9) | 15 (8.4) | 8 (4.5) | 4 (2.3) | |
| Streptococci (58) | 46 (79.3) | 3 (5.2) | 6 (10.3) | 1 (1.7) | - | - | 2 (3.5) | |
| Stenotrophomonas (54) | 28 (51.8) | 22 (40.7) | 3 (5.6) | - | 1 (1.9) | - | - | |
| H. influenzae (29) | 17 (58.6) | 4 (13.8) | 4 (13.8) | 2 (6.9) | 1 (3.4) | 1 (3.4) | - | |
| Urinary isolates | E. coli (877) | 204 (23.2) | 102 (11.6) | 162 (18.5) | 173 (19.7) | 90 (10.3) | 44 (5.0) | 102 (11.6) |
| Klebsiella (341) | 68 (19.9) | 34 (10.0) | 105 (30.8) | 64 (18.8) | 35 (10.3) | 14 (4.1) | 21 (6.2) | |
| Enterobacter (39) | 15 (38.5) | 7 (17.9) | 7 (17.9) | 4 (10.3) | 2 (5.1) | 3 (7.7) | 1 (2.6) | |
| A. baumannii (93) | 32 (34.4) | 16 (17.2) | 14 (15.1) | 8 (8.6) | 7 (7.5) | 4 (4.3) | 12 (12.9) | |
| Enterococci (270) | 122 (45.1) | 75 (27.8) | 33 (12.2) | 13 (4.8) | 8 (2.9) | 9 (3.3) | 10 (3.7) | |
| Proteus sp (61) | 18 (29.5) | 12 (19.7) | 10 (16.4) | 6 (9.8) | 7 (11.5) | 5 (8.2) | 3 (4.9) | |
| Pseudomonas sp (182) | 121 (66.5) | 24 (13.2) | 12 (6.6) | 7 (3.8) | 7 (3.8) | 0 | 11 (6.0) | |
| S. aureus (64) | 19 (29.7) | 24 (37.5) | 10 (15.6) | 5 (7.8) | 3 (4.7) | 3 (4.7) | - | |
| Miscellaneous isolates | E. coli (269) | 61 (22.7) | 39 (14.5) | 53 (19.7) | 45 (16.7) | 33 (12.3) | 19 (7.1) | 19 (7.1) |
| Klebsiella (204) | 44 (21.6) | 23 (11.3) | 63 (30.9) | 31 (15.2) | 22 (10.8) | 10 (4.9) | 11 (5.4) | |
| Enterobacter (66) | 39 (59.1) | 10 (15.2) | 10 (15.2) | 1 (1.5) | 3 (4.6) | 0 | 3 (4.6) | |
| A. baumannii (190) | 54 (28.4) | 36 (18.9) | 13 (6.8) | 15 (7.9) | 9 (4.7) | 14 (7.4) | 49 (25.8) | |
| Enterococci (122) | 58 (47.5) | 33 (27.0) | 13 (10.7) | 9 (7.4) | 4 (3.3) | 4 (3.2) | 1 (0.8) | |
| Pseudomonas (289) | 190 (65.7) | 28 (9.7) | 10 (3.5) | 15 (5.2) | 19 (6.6) | 5 (1.7) | 12 (4.2) | |
| Proteus (114) | 44 (38.6) | 21 (18.4) | 21 (18.4) | 12 (10.5) | 12 (10.5) | 3 (2.6) | 1 (0.9) | |
| S. aureus (314) | 149 (47.5) | 55 (17.5) | 32 (10.2) | 20 (6.4) | 36 (11.5) | 13 (4.1) | 9 (2.9) | |
| Citrobacter (18) | 7 (38.9) | 4 (22.2) | 2 (11.1) | 1 (5.6) | 2 (11.1) | 1 (11.1) | 1 (5.6) | |
| Percentage of resistant bacteria is shown in parenthesis | ||||||||
Table 2. Resistance profile of bacterial isolates against number of antimicrobials.
Comparing the results of antimicrobials susceptibility tests of all the bacterial isolates between susceptible to all antimicrobials and resistant to one or more antimicrobials provided us a better picture of resistance profiles of the isolates (Table 3). Among respiratory A. baumannii, 35.7% were susceptible to all antimicrobials whereas 64.3% were found resistant to either one or more antimicrobials simultaneously. Eleven out of total 48 E. coli from respiratory cultures were susceptible to all antimicrobials tested while remaining 37 were found exhibiting resistance to one or more antimicrobials at a time (Table 3). Similarly, 80% Klebsiella were found resistant toward one or more than one antimicrobials. In isolates of urinary sources, 76.7% E. coli were resistant to one or more antimicrobials simultaneously. Percentages of 80.1% Klebsiella, 70.3% S. aureus and 70.5% Proteus species from urine cultures were found resistant to one or more antimicrobials at a time. A similar result was also observed in bacteria isolated from miscellaneous sources. We further, analyzed the resistance profile of all isolates from different groups after combining their results, where we have found that 67.2% A. baumannii isolates were resistant to one or more antimicrobials. Among E. coli isolates, 23.1% were sensitive to all antimicrobials tested while remaining 76.9% were found showing resistance to one or more antimicrobials simultaneously (Table 3). Similarly, majority of Klebsiella, S. aureus, Enterococci and Proteus species were found resistant to one or more antimicrobials at a time. However, majority (63.2%) of Pseudomonas isolates were susceptible to all antimicrobials tested.
| Group | Bacteria | Susceptible to all antimicrobials (%) | Resistant to one or more antimicrobials (%) |
|---|---|---|---|
| Respiratory isolates | A. baumanii (244) | 87 (35.7) | 157 (64.3) |
| E. coli (48) | 11 (22.9) | 37 (77.1) | |
| Enterobacter (36) | 16 (44.4) | 20 (55.6) | |
| H. influenzae (29) | 17 (58.6) | 12 (41.4) | |
| Klebsiella (135) | 27 (20.0) | 108 (80.0) | |
| Pseudomonas (300) | 176 (58.7) | 124 (41.3) | |
| Streptococci (58) | 46 (79.3) | 12 (20.7) | |
| S. aureus (178) | 93 (52.2) | 85 (47.8) | |
| Stenotrophomonas (54) | 28 (51.9) | 26 (48.1) | |
| Urinary isolates | E. coli (877) | 204 (23.3) | 673 (76.7) |
| Enterococci (270) | 122 (45.2) | 148 (54.8) | |
| A. baumanii (93) | 32 (34.4) | 61 (65.6) | |
| Enterobacter (39) | 15 (38.5) | 24 (61.5) | |
| Klebsiella (341) | 68 (19.9) | 273 (80.1) | |
| Proteus sp (61) | 18 (29.5) | 43 (70.5) | |
| Pseudomonas sp (182) | 121 (66.5) | 61 (33.5) | |
| S. aureus (64) | 19 (29.7) | 45 (70.3) | |
| Miscellaneous isolates | Enterococci (122) | 58 (47.5) | 64 (52.5) |
| Klebsiella (204) | 44 (21.6) | 160 (78.4) | |
| Pseudomonas (289) | 190 (65.7) | 99 (34.3) | |
| Enterobacter (66) | 39 (59.1) | 27 (40.9) | |
| Proteus (114) | 44 (38.6) | 70 (61.4) | |
| A. baumanii (190) | 54 (28.4) | 136 (71.6) | |
| Citrobacter (18) | 7 (38.9) | 11 (61.1) | |
| E. coli (269) | 61 (22.7) | 208 (77.3) | |
| S. aureus (314) | 149 (47.5) | 165 (52.5) | |
| All isolates combined | A. baumanii (527) | 173 (32.8) | 354 (67.2) |
| E. coli (1194) | 276 (23.1) | 918 (76.9) | |
| Enterobacter (141) | 70 (49.7) | 71 (50.4) | |
| Klebsiella (680) | 139 (20.4) | 541 (79.6) | |
| Pseudomonas (771) | 487 (63.2) | 284 (36.8) | |
| S. aureus (556) | 261 (46.9) | 295 (53.1) | |
| Enterococci (392) | 180 (45.9) | 212 (54.1) | |
| Proteus (175) | 62 (35.4) | 113 (64.6) |
Table 3. Comparison of antimicrobials sensitivity versus resistance among bacterial isolates recovered from different sources.
Incidence of ESBL producers
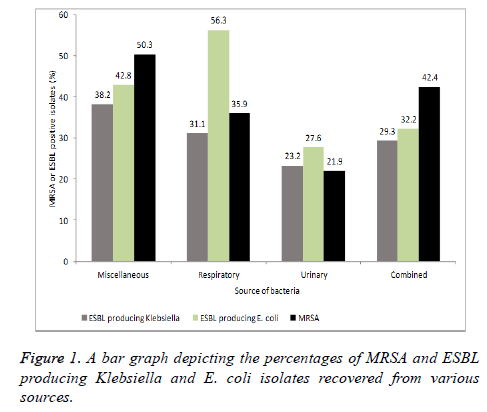
Klebsiella and E. coli isolates were also screened for the production of ESBL producers. The ESBLs are the enzymes, which are responsible for the resistance to beta-lactam antibiotics. In the clinical isolates from respiratory sources; 56.3% E. coli and 31.1% Klebsiella isolates found positive for ESBLs. Nearly a quarter of Klebsiella and E. coli isolates from urinary sources were positive for ESBLs production. Analyzing the results after combining all three different groups revealed that 29.3% Klebsiella and 32.2% E. coli were positive for ESBLs (Figure 1).
Incidence of MRSA
S. aureus isolates were subjected to the resistance profiling against oxacillin. A total of 50.3% S. aureus isolates from miscellaneous sources were found exhibiting resistance against oxacillin whereas 35.9% were classified as MRSA among respiratory sources. However, fewer number of MRSA isolates were observed in urine cultures (Figure 1). Combining all the results revealed that overall 42.4% S. aureus isolates were MRSA.
Discussion
The present study was undertaken to analyze the prevalence of antimicrobials resistance among bacterial isolates from various sources like blood, urine, respiratory, skin, wound, and stool. In the study, we have found nearly 50% of E. coli isolates from urinary sources were resistant to SXT and another 46.5% were resistant to PRL. A study from Najran region of Saudi Arabia had reported that 31.1% of E. coli from urine was resistant to multiple drugs [13]. In another study, 36.7% isolates of E. coli were observed resistant to ampicillin, amoxicillin, carbenicillin and piperacillin [9]. Kresken and colleagues [14] have observed that 42.9% of 499 E. coli isolates from urinary tract were resistant to amoxicillin followed by AMC (32.7%) and SXT (30.9%). An observational study in Ireland found that 87.9% of 366 E. coli isolates from urine were resistant to nalidixic acid, 82.7% were resistant to ciprofloxacin and 81.4% were reported resistant to trimethoprim [15].
In the current study, we have found 59.3% of 135 Klebsiella isolates from respiratory sources were resistant to AMP and PRL. Other bacterial species isolated from respiratory sources like Enterobacter, A. baumannii, Pseudomonas were mostly sensitive to the antimicrobials tested. A German study has reported the prevalence of different bacterial species from respiratory tract infections and found that out of 438 Klebsiella isolates, 32.2% and 15.3% were resistant to AMC and cefuroxime respectively. They further reported that 14.4% of S. aureus isolates (n=485) were resistant to AMC [16]. Our study has found that most of the A. baumannii isolates (n=244) were sensitive to the antimicrobials tested, which are contradictory with the findings to a study from Vietnam where A. baumannii isolates were resistant to AMC and SXT (100%), TZP (95.2%), cefotaxime and ceftazidime (98.4%). But they have included only 63 A. baumannii isolates in their study, which is quite a small number [17]. We have found 53 out of 178 respiratory S. aureus isolates resistant to OX followed by erythromycin (23.0%). However, most of our S. aureus isolates were found sensitive to other antimicrobials tested. These results are contradictory to the findings of a large surveillance study conducted in Japan where most of the 206 S. aureus isolates were found exhibiting resistance to the antimicrobials tested including 50.5% and 62.0% were resistant to oxacillin and erythromycin respectively [18]. In the current study, Klebsiella isolates from respiratory sources were equally resistant to AMP and PRL (59.5%). However, Pseudomonas isolates, which were 300 in number, were mostly susceptible to the majority of the antimicrobials tested. Highest number of Pseudomonas isolates were observed resistant against PRL (19.7%) followed by 18.3% to TZP. Similar to our findings regarding Klebsiella was also reported from Japan [18], where 80.6% of 136 Klebsiella isolates were shown resistant to AMP. Moreover, their 136 Klebsiella isolates were susceptible to the other antimicrobials tested, the trend we have also observed in our study. Regarding Pseudomonas, our results are in agreement with their findings.
Klebsiella and E. coli isolates were investigated for the production of ESBLs. Of the 135 respiratory Klebsiella isolates, 31.1% were found positive for ESBLs and among E. coli, 56.25% were positive for the same enzyme. There are varied reports with regard to the prevalence of ESBL producing Klebsiella and E. coli isolates from different parts of the world. The prevalence of ESBLs positive respiratory Klebsiella isolates in Indonesia has been reported to be 58.5%, in China it was 38.8% whereas in Singapore and Taiwan the prevalence of ESBLs in Klebsiella isolates was observed at 7.5% and 2% respectively [19]. A study from Saudi Arabia regarding the prevalence pattern of ESBL producing strains found that the incidence of ESBLs was 48.4% in K. pneumoniae followed by 15.8% in E. coli [10]. In another Saudi study, it was reported that 24.8% E. coli and 30.5% of K. pneumoniae were positive for ESBLs production [20]. The present study found that out of 877 E. coli isolates from urine, 27.6% were positive for ESBL production. A study from Germany on urinary isolates reported only 8% of 499 E. coli were positive for ESBLs [14]. An investigation from Asia- Pacific region found 36.52% of 1361 E. coli isolates from urine were positive for ESBL production [21]. In our study, the prevalence of ESBL producing Klebsiella and E. coli isolates after combining all the isolates from three different categories, revealed that 29.3% of 680 Klebsiella isolates were positive for ESBLs whereas 32.2% out of 1194 E. coli were ESBL positive. These results are comparable to a report from China where 42.3% of 634 E. coli and 31.7% of 606 Klebsiella isolates were found possessing ESBLs [22].
With regard to the prevalence of methicillin-resistance in S. aureus isolates, we found 35.9% and 21.9% MRSA isolates in respiratory and urinary sources respectively. After combining all S. aureus isolates from miscellaneous, respiratory and urinary sources, the pooled prevalence of MRSA was found to be 42.4% in the current study from Western region of Saudi Arabia. A previous study from the same hospital, King Abdulaziz University hospital, Jeddah, Saudi Arabia reported only 10.2% MRSA isolates but they investigated only 98 S. aureus isolates [23]. In another study, the prevalence of MRSA was reported to be 39.5% where infection was commonly associated with wound, skin, and soft tissues [11]. A retrospective countrywide analysis in Saudi Arabia on 22,793 S. aureus strains, found 35.6% of the strains resistant to methicillin. Further, comparative analysis on prevalence of MRSA was shown to be ranging from 5.97% in Dhahran to 94% in Riyadh cities [12]. A Japanese study found that among 206 respiratory S. aureus isolates, 50.5% were MRSA [18]. However, a German study reported only 16.3% MRSA out of total 485 respiratory S. aureus isolates [16]. An earlier study about the prevalence of MRSA in Saudi Arabia reported that 29.9% of S. aureus isolates were methicillin-resistant [24].
The study has provided us the information about the antimicrobial susceptibility patterns in Escherichia coli, Klebsiella and other bacterial pathogens recovered from Western Region of Saudi Arabia. The study has also provided information regarding the prevalence of ESBL production and MRSA isolates in the region. The present surveillance data and antimicrobials resistance profiles of different groups of bacteria will be helpful to microbiologists and clinicians in recommending the best possible antimicrobial drugs based on the resistance pattern for the treatment of various infections in Western Region of Saudi Arabia. There is a need to check the spread of antimicrobial resistance in pathogenic bacteria, which can be achieved through judicious use of antimicrobial agents. Further, surveillance studies must be continued to determine the actual pattern of the bacterial resistance to different antimicrobial drugs.
Acknowledgment
This work was supported by Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No (141-798-D1435). The authors, therefore, gratefully acknowledge the DSR technical and financial support.
References
- Cosgrove SE, Carmeli Y. The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis 2003; 36: 1433-1437.
- Hoffmann K, Wagner G, Apfalter P, Maier M. Antibiotic resistance in primary care in Austria-a systematic review of scientific and grey literature. BMC Infect Dis 2011.
- Theuretzbacher U. Accelerating resistance, inadequate antibacterial drug pipelines and international responses. Int J Antimicrob Agents 2012; 39: 295-299.
- Bradley JS, Guidos R, Baragona S, Bartlett JG, Rubinstein E, Zhanel GG. Anti-infective research and development-problems, challenges, and solutions. Lancet Infect Dis 2007; 7: 68-78.
- Jones RN, Castanheira M, Hu B, Ni Y, Lin SSF, Mendes RE. Update of contemporary antimicrobial resistance rates across China: reference testing results for 12 medical centers (2011). Diag Microbiol Infect Dis 2013; 77: 258-266.
- Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006; 368: 874-885.
- Chu VH, Crosslin DR, Friedman JY, Reed SD, Cabell CH, Griffiths RI. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am J Med 2005.
- Kumari N, Mohapatra TM, Singh YI. Prevalence of Methicillin-resistant Staphylococcus aureus (MRSA) in a Tertiary-Care Hospital in Eastern Nepal. J Nepal Med Assoc 2008; 47: 53-56.
- Halawani EM. Beta-lactam antibiotic resistance in Escherichia coli commensal faecal flora of healthy population in Taif, Saudi Arabia. African J Microbiol Res 2011; 5: 73-78.
- El-Khizzi NA, Bakheshwain SM. Prevalence of extended-spectrum beta-lactamases among Enterobacteriaceae isolated from blood culture in a tertiary care hospital. Saudi Med J 2006; 27: 37-40.
- El-Amin NM, Faidah HS. Methicillin-resistant Staphylococcus aureus in the western region of Saudi Arabia: prevalence and antibiotic susceptibility pattern. Annals Saudi Med 2012; 32: 513-516.
- Yousef SA, Mahmoud SY, Eihab MT. Prevalence of methicillin-resistant Staphylococcus aureus in Saudi Arabia: Systemic review and meta-analysis. African J Clin Exp Microbiol 2013; 14: 146-154.
- Masoud E, Mahdy M, Esmat A. Bacterial prevalence and resistance to antimicrobial agents in Southwest, Saudi Arabia. Egypt Acad J biolog Sci 2011; 3: 105-111.
- Kresken M, Pfeifer Y, Hafner D, Wresch R, Körber-Irrgang B, Working Party ‘Antimicrobial Resistance’ of the Paul-Ehrlich-Society for C. Occurrence of multidrug resistance to oral antibiotics among Escherichia coli urine isolates from outpatient departments in Germany: extended-spectrum β-lactamases and the role of fosfomycin. Int J Antimicrob Agents 2014; 44: 295-300.
- Fennell J, Vellinga A, Hanahoe B, Morris D, Boyle F, Higgins F. Increasing prevalence of ESBL production among Irish clinical Enterobacteriaceae from 2004 to 2008: an observational study. BMC Infect Dis 2012.
- Jacobs E, Dalhoff A, Korfmann G. Susceptibility patterns of bacterial isolates from hospitalised patients with respiratory tract infections (MOXIAKTIV Study). Int J Antimicrob Agents 2009; 33: 52-57.
- Van TD, Dinh Q-D, Vu PD, Nguyen TV, Pham CV, Dao TT. Antibiotic susceptibility and molecular epidemiology of Acinetobacter calcoaceticus-baumannii complex strains isolated from a referral hospital in northern Vietnam. J Global Antimicrob Resist 2014; 2: 318-321.
- Yanagihara K, Kadota J, Aoki N, Matsumoto T, Yoshida M, Yagisawa M. Nationwide surveillance of bacterial respiratory pathogens conducted by the surveillance committee of Japanese Society of Chemotherapy, the Japanese Association for Infectious Diseases, and the Japanese Society for Clinical Microbiology in 2010: General view of the pathogens' antibacterial susceptibility. J Infect Chemother 2010.
- Wang H, Chen M, Xu Y, Sun H, Yang Q, Hu Y. Antimicrobial susceptibility of bacterial pathogens associated with community-acquired respiratory tract infections in Asia: report from the Community-Acquired Respiratory Tract Infection Pathogen Surveillance (CARTIPS) study, 2009-2010. Int J Antimicrob Agents 2011; 38: 376-383.
- Shibl AM, Al-Agamy MH, Khubnani H, Senok AC, Tawfik AF, Livermore DM. High prevalence of acquired quinolone-resistance genes among Enterobacteriaceae from Saudi Arabia with CTX-M-15 β-lactamase. Diagnost Microbiol Infect Dis 2012; 73: 350-353.
- Lu PL, Liu YC, Toh HS, Lee YL, Liu YM, Ho CM. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009-2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int J Antimicrob Agents 2012; 40: S37-43.
- Xiao Y, Wei Z, Shen P, Ji J, Sun Z, Yu H. Bacterial-resistance among outpatients of county hospitals in China: significant geographic distinctions and minor differences between central cities. Microbes and Infection / Institut Pasteur 2015.
- Helmi N, Zaman R, Aly M. Prevalence of Gram-positive bacteria in Jeddah, Kingdom of Saudi Arabia: study of antimicrobial resistance patterns and molecular typing. Int J Pharm Bio Sci 2013; 4: 1231-1245.
- Aly M, Balkhy HH. The prevalence of antimicrobial resistance in clinical isolates from Gulf Corporation Council countries. Antimicrob Resist Infect Control 2012.