ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 4
2-Methoxyestradiol-bis-sulphamate induces apoptosis and autophagy in an oesophageal carcinoma (SNO) cell line.
Department of Physiology, University of Pretoria, Private Bag X323, Arcadia, PRETORIA, 0007, South Africa
- *Corresponding Author:
- Annie Joubert
Department of Physiology
University of Pretoria, Private Bag X323, Arcadia
PRETORIA, 0007
South Africa
Accepted date: July 17 2012
2-Methoxyestradiol (2ME2) is a natural metabolite of 17-β-estradiol exerting both antiproliferative and antiangiogenic characteristics. Due to limited bioavailability, analogues of 2ME2 were designed, synthesized and evaluated for improved in vitro antiproliferative activity as well as bioavailability. 2-Methoxyestradiol-bis-sulphamate (2-MeOE2bisMATE) is an analogue of 2ME2 which was produced by sulphamoylation of the two hydroxyl groups on carbons 3 and 17. The aim of this in vitro study was to evaluate the influence of 2- MeOE2bisMATE on morphology, apoptosis and autophagy induction in an oesophageal carcinoma (SNO) cell line by means of transmission electron microscopy (TEM) and flow cytometry (cyto-ID and LC3 autophagy detection assays). In 2-MeOE2bisMATE-treated cells, features of both apoptosis and autophagy such as the presence of apoptotic bodies and autophagy vesicles were observed using the transmission electron microscopy. A significant increase in the quantity of autophagy vesicles and LC3 levels were observed in 2- MEOE2bisMATE-treated cells. This in vitro study demonstrates that 2-MeOE2bisMATE induces both apoptosis and autophagy which was revealed by the increase in apoptosis- and autophagy-associated morphology, as well as by an increase in the measure of autophagy vesicles and LC3 levels in SNO cells. From data observed from this study, 2-MeOE2bisMATE is shown to be a potential anticancer agent; further in vitro research is, however, warranted.
Keywords
2-Methoxyestradiol-bis-sulphamate, Transmission electron microscope, Autophagy, Apoptosis
Introduction
During the previous few decades 2-methoxyestradiol (2ME2), the biological metabolite of 17β-estradiol, has been shown to be a potential anticancer drug with the ability to inhibit proliferation and angiogenesis in vitro and in vivo [1,2,3,4,5,6,7]. The antiproliferative activity of 2ME2 was found to be time- and concentrationdependent, as well as estrogen receptor (ER) independent [6,8]. 2ME2 binds to the colchicine binding site on tubulin, causing cells to undergo mitotic arrest which subsequently leads to the induction of cell death [3,9,10]. Although 2ME2 has been shown to have antiproliferative and anti-angiogenic properties, human clinical trials for the treatment of breast cancer, prostate cancer and multiple myeloma have shown that high oral doses of 2ME2 (registered as Panzem® by Entremed, Inc. (Rockville, MD)) are needed per day in order to significantly reduce the growth of certain tumours [11,12,13,14]. 2ME2 has a low bioavailability and it is rapidly inactivated by 17β- hydroxysteroid dehydrogenase type 2 and by conjugation of both 3- and 17-hydroxyl moieties to form glucuronides [15,16,17].
In an attempt to address the problem of low solubility and bioavailability, new nanocrystal dispersion (NCD) formulation of 2ME2 has been developed [18]. Although a higher plasma levels of about 3-4% was observed in the nanoparticulate dispersions when compared to the usual 2ME2 formulations, the bioavailability still remains very low [18].
2-Methoxyestradiol-bis-sulphamate (2-MeOE2bisMATE) is a bis-sulphamoylated derivative of 2ME2, which is currently being researched as a potential anticancer drug [19,20]. Recent in vitro studies from our laboratory and from other researchers, have found that 2-MeOE2bisMATE inhibits cell growth in estrogen receptor negative and positive cells, as well as multiple drug resistant cell lines [21,22,23,24]. 2-MeOE2bisMATE inhibited the growth of human breast adenocarcinoma ER- cell line (MDAMB- 231) with an IC50 of 0.33μM [24]. Suzuki et al. (2003) showed that treatment of MCF-7 cells resistant to doxorubicin and mitoxantrone with 1μM of 2- MeOE2bisMATE inhibited cell proliferation [25].
In view of the fact that the exact mechanism of action of 2-MeOE2bisMATE is still not well defined especially in oesophageal carcinoma cells, this in vitro study was conducted to further contribute to the action mechanism of 2- MeOE2bisMATE in these cells.
Materials and Methods
Cell line
The SNO oesophageal carcinoma cell line is described as a non-keratinizing squamous epithelial cell. The SNO cell line was purchased from Highveld Biological Ltd. (Pty) (Sandringham, South Africa).
Compound and reagents
Since 2-MeOE2bisMATE is not commercially available, the compound was synthesized by Prof. Vleggaar from the Department of Chemistry (University of Pretoria, Pretoria, South Africa). All required reagents of cell culture analytical grade were purchased from Sigma (St. Louis, United States of America) unless otherwise specified. Cyto-ID autophagy detection kit and the Rabbit polyclonal anti-LC3B conjugated to DayLight 488 were bought supplied by BIOCOM biotech (Pty) Ltd. (Clubview, South Africa).
Cell culture
Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heatinactivated fetal calf serum (FCS) and 100 U/ml penicillin G, 100 μg/ml streptomycin and 250 μg/l fungizone. Cells were cultured in 25 cm2 tissue culture flasks at 37°C in a humidified atmosphere containing 5% CO2 and left over night for attachment. A stock solution of 2- MeOE2bisMATE with a concentration of 2.0x103M was dissolved in dimethyl sulphoxide (DMSO) and kept at - 20°C. Appropriated controls were included for each experiment. All experiments included control samples in the form of cells propagated in growth medium, cells treated with the same volume of DMSO used to expose cells with 2-MeOE2bisMATE, as well as positive controls for apoptosis (0.1μg/ml actinomycin D) and autophagy (tamoxifen 20μM). A concentration of 0.4μM and an exposure period of 24 hours were selected; since it was observed in our prior study that it is in this range that 2-MeOE2bisMATE significantly decreased cell proliferation of SNO cells [27].
Transmission electron microscopy
TEM allows imaging of inside cell structures with a resolution power of about 0.1-0.4 nm, therefore this study was conducted in order to study the effects induced by 2-MeOE2bisMATE on SNO cells [28]. SNO cells were seeded at a density of 500 000 cells per 25cm2 flask. After 24 hours of cell attachment, the medium was discarded and the cells were exposed to 2-MeOE2bisMATE, and appropriate controls were included. The experiment was terminated by trypsinizing the cells, fixing them in 2.5% glutaraldhyde in 0.075M phosphate buffer (pH 7.4) and afterwards washing them with 0.075M phosphate buffer. Subsequently, cells were fixed in 0.25% aqueous osmium solution and rinsed with increasing concentrations of ethanol (30%, 50%, 70%, 90%, and 100%) and embedded in Quetol resin. Ultra-thin sections were prepared using a microtome and then they were mounted on a copper grid. Samples were contrasted with 4% uranyl acetate and Reynolds’ lead citrate and viewed with a Multi-purpose Philips 301 TEM (Electron Microscopy Unit, Pretoria, South Africa).
Cyto-ID autophagy detection assay
Cyto-ID Autophagy Detection Kit measures autophagic vacuoles using a stain that selectively labels autophagic vacuoles. This method permits for staining of preautophagosomes, autophagosomes, and autolysosomes, therefore offering quantitative approach for detecting autophagy [29]. Exponentially growing SNO cells were seeded into 25cm2 flasks at a density of 500 000 each. After a 24 hour incubation period at 37°C, cells were exposed to 2-MeOE2bisMATE and to appropriate controls for 24 hours. Following compound treatment, the cells were washed once in PBS and resuspended in a dilution of Cyto-ID Green autophagy detection reagent and incubated at 37°C for 30 minutes. Subsequently, the cells were analyzed using CXP software (Beckman Coulter South Africa (Pty) Ltd). Cyto-ID Green autophagy reagent was measured in the fluorescent channel (Fl1 Log) using Cyflogic 1.2.1 software (Perttu Terho & Cyflo Ltd).
Autophagy detection: rabbit polyclonal anti-LC3B conjugated to DyLight 488
The LC3 antibody detection method was utilized to determine whether 2-MeOE2bisMATE induces autophagy in SNO cells [30]. Exponentially growing SNO cells were seeded at 500 000 cells per 25cm2 flask. After 24 hours of attachment, medium was discarded and cells were exposed to 2-MeOE2bisMATE. Following incubation the cells were trypsinized and washed with cold PBS. Cells were fixed with 0.01% formaldehyde in PBS for 10 minutes, pelleted and were then resuspendend in 200μl PBS. To further fix and permeabilize the cells, 1ml of ice-cold methanol (-20°C) was added to the solution in a dropwise manner. Cells were then pelleted and washed twice with cold PBS. After washing, the cells were pelleted and 0.5ml of the antibody cocktail was added and then incubated for 2 hours at 4°C in the dark. Following 2 hours of incubation, the cells were washed trice with a wash buffer (PBS/0.05% Triton X-100/1%BSA) and then analyzed with CXP software (Beckman Coulter South Africa (Pty) Ltd). Data from 30 000 events was analyzed using Cyflogic 1.2.1 software ((Perttu Terho & Cyflo Ltd).
Statistical analysis
Autophagy detection analysis (anti-LC3B & cyto-ID) assays were analyzed quantitatively, while TEM was analyzed qualitatively. The ANOVA students’ t-test were used to determine the analytical variation in experimental procedures and biological variations within each experiment. For flow cytometric data no less than 30 000 events were counted for each sample and three independent experiments were conducted. Data produced were analysed using Cyflogic 1.2.1 (Perttu Terho & Cyflo Ltd).
Results
Transmission electron microscopy
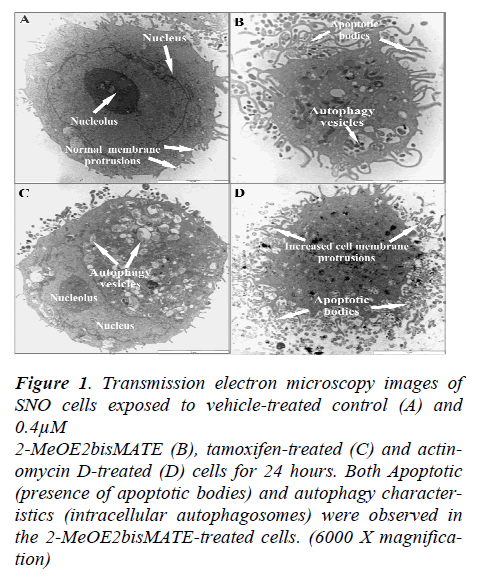
Transmission electron microscope (TEM) allows for a detailed study of intracellular cell structures. Results from TEM showed an increase in the presence of apoptotic bodies and autophagosomes in 2-MeOE2bisMATEtreated cells (Figure 1C). In addition, 2-MeOE2bis- MATE- treated cells were shrunken in size with increased cell membrane blebbing indicative of cellular stress.
Figure 1. Transmission electron microscopy images of SNO cells exposed to vehicle-treated control (A) and 0.4μM 2-MeOE2bisMATE (B), tamoxifen-treated (C) and actinomycin D-treated (D) cells for 24 hours. Both Apoptotic (presence of apoptotic bodies) and autophagy characteristics (intracellular autophagosomes) were observed in the 2-MeOE2bisMATE-treated cells. (6000 X magnification)
Cyto-ID autophagy detection assay
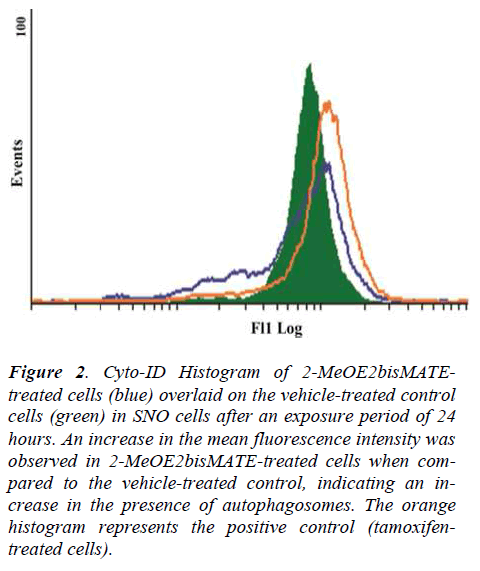
The cyto-ID autophagy detection assay was conducted to test for the presence of autophagosomes in 2-MeOE2bisMATE-treated SNO cells. Results showed that exposure of SNO cells to 2-MeOE2bisMATE resulted in an increase in the presence of autophagosomes, as demonstrated by the shift to the right on the histogram, when compared to the vehicle-treated control (Figure 2).
Figure 2. Cyto-ID Histogram of 2-MeOE2bisMATEtreated cells (blue) overlaid on the vehicle-treated control cells (green) in SNO cells after an exposure period of 24 hours. An increase in the mean fluorescence intensity was observed in 2-MeOE2bisMATE-treated cells when compared to the vehicle-treated control, indicating an increase in the presence of autophagosomes. The orange histogram represents the positive control (tamoxifentreated cells).
Tamoxifen was used as a positive control agent known for induction of autophagy.
Autophagy detection: rabbit polyclonal anti-LC3B conjugated to DyLight 488
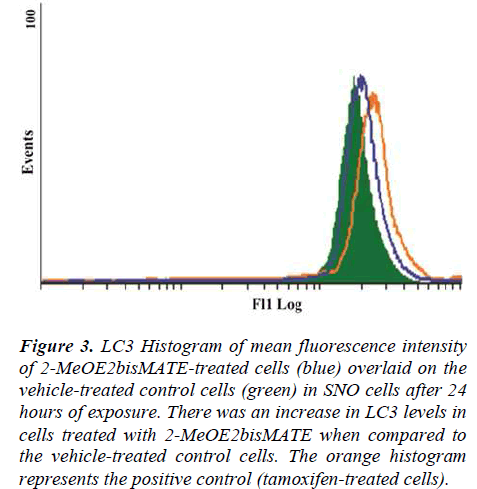
A conjugated rabbit polyclonal anti-LC3B antibody assay was used to test for autophagy. The exposure of SNO cells to 2-MeOE2bisMATE resulted in an increase in LC3 levels, as seen by the shift to the right on the histogram, when compared to the vehicle-treated control (Figure 3).
Figure 3. LC3 Histogram of mean fluorescence intensity of 2-MeOE2bisMATE-treated cells (blue) overlaid on the vehicle-treated control cells (green) in SNO cells after 24 hours of exposure. There was an increase in LC3 levels in cells treated with 2-MeOE2bisMATE when compared to the vehicle-treated control cells. The orange histogram represents the positive control (tamoxifen-treated cells).
Discussion
In this study the in vitro effects of 2-MeOE2bisMATE on cell morphology and its potential to induce cell death in oesophageal carcinoma cells were investigated. TEM was used to analyse the intracellular structures of the SNO cells after 24 hours of exposure to 2-MeOE2bisMATE. TEM results revealed significant morphological changes that were characteristic of apoptosis (apoptotic bodies) and autophagy (autophagosomes). Previous studies conducted in our laboratory demonstrated that 0.4μM of 2- MeOE2bisMATE induced both apoptosis and autophagy in human breast epithelial cancer (MCF-7) cells after 24 hours of incubation [22, 26]. Our TEM results suggest that 2-MeOE2bisMATE induces cell death by both apoptosis and autophagy in SNO cells after 24 hours of exposure. These results were also observed in our triple staining investigations which revealed an extensive staining of SNO cells in hoechst and acridine orange, suggesting that both apoptosis and autophagy were induced by 2- MeOE2bisMATE [27].
.Results obtained using TEM as well as our previous morphological studies revealed autophagy was induced by 2- MeOE2bisMATE in SNO cells. Autophagy is a process whereby a cell degrades its intracellular components inside lysosomes [31]. It is one of the cell’s two major pathways through which proteins are degraded and it is the only pathway known for degrading cellular organelles [32-34]. In normal cells, autophagy maintains cellular homeostasis by removing excessive proteins and damaged organelles [32]. Autophagy can be induced by various conditions such as starvation, growth factor withdrawal and stressors [32,35,36]. Generally, autophagy is a mechanism of survival for cells during periods of stress and starvation, but there are times where autophagy can progress to cell death [37].
Lorin et al. (2009) demonstrated that 2ME2 induces both apoptosis and autophagy of ewing sarcoma cells, similarly Azad et al. (2009) demonstrated that treatment of human glioblastoma astrocytoma epithelial-like cell line (U87), human cervical adenocarcinoma cells (HeLa), and transformed human embryonic kidney cells (HEK293) with 2ME2 induced both types of cell deaths [38,39]. In this study the cyto-ID autophagy detection and the conjugated rabbit polyclonal anti-LC3B antibody assays were conducted to confirm our previous results of autophagy induction in SNO cells by 2-MeOE2bisMATE. These assays revealed an increase in the presence of autophagosomes and in the LC3 levels.
The occurrence of both apoptosis and autophagy suggests that there is a crosstalk between these two types of cell deaths. Studies on cell deaths have demonstrated that there are some common signaling pathways between apoptosis and autophagy [40-41]. These include signaling pathways such as PI3k/AKT, regulatory genes such as p53 and p19ARF, as well as some of the basic machinery that execute the cell death programs (such as Atg5, Bcl-2) [42,43]. It has been shown that Bcl-2 and Bcl-xL, the anti-apoptosis proteins, bind to Beclin 1 thus resulting in the inhibiting Beclin 1-mediated autophagy [44,45]. Therefore, stimuli that lead to the activation or suppression of these genes or signaling pathways can influence both apoptosis and autophagy [42]. The exact network that controls the crosstalk between apoptosis and autophagy still needs to be clarified.
Conclusion
In conclusion, this in vitro study revealed that 2- MeOE2bisMATE induced both apoptosis and autophagy in SNO cells. Results from this study suggest that there may be a crosstalk between apoptosis and autophagy. This in vitro study revealed novel insights on the action mechanism of 2-MeOE2bisMATE in oesophageal SNO carcinoma cells and contributes to the understanding of the mechanism of action of 2-MeOE2bisMATE in these cells. Future studies on gene and protein expression, as well as on signaling molecules involved in the crosstalk, will be conducted to further understand the mechanism of action of 2-MeOE2bisMATE.
Acknowledgements
This study was supported by grants awarded to Professor AM Joubert (University of Pretoria) from the National Research Foundation (NRF), Cancer Association of South Africa (AK246), Medical Research Council (AG374, AK076), Struwig Germishuysen Trust (AJ038) and Research Committee of the University of Pretoria (Pretoria, South Africa). Flow cytometry and electron microscope were conducted at the Department of Pharmacology and at the Electron Microscopy Unit, respectively (University of Pretoria, Pretoria, South Africa).
References
- Pribluda VS, Gubish ER, LaVallee TM, et al. 2- Methoxyestradiol: An endogenous anti angiogenic and anti-proliferative drug candidate. Cancer Metastasis Rev 2000; 19: 173-179.
- Fotsis T, Zhang Y, Pepper MS, et al. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature 1994; 368: 237-239.
- Bhati R, Gokmen-Polar Y, Sledge GW, et al. 2- Methoxyestradiol inhibits the anaphase-promoting complex and protein translation in human breast cancer cells. Cancer Res 2007; 67: 702-708.
- Dingli D, Timm M, Russell SJ, et al. Promising preclinical activity of 2-methoxyestradiol in multiple myeloma. Clin Cancer Res 2002; 8: 3948-3954.
- Fukui M, Zhu BT. Mechanism of 2-methoxyestradiolinduced apoptosis and growth arrest in human breast cancer cells. Mol Carcinog 2009; 48: 66-78.
- Van Zijl C, Lottering ML, Steffens F, et al. In vitro effects of 2-methoxyestradiol on MCF-12A and MCF-7 cell growth, morphology and mitotic spindle formation. Cell Biochem Funct. 2008; 26: 632-642.
- Thaver V, Lottering M, Van Papendorp D, et al. In vitro effects of 2-methoxyestradiol on cell numbers, morphology, cell cycle progression, and apoptosis induction in oesophageal carcinoma cells. Cell Biochem Funct 2009; 24: 205-210.
- LaVallee TM, Zhan XH, Herbstritt CJ, et al. 2- Methoxyestradiol Inhibits Proliferation and Induces Apoptosis Independently of Estrogen Receptors α and β. Cancer Res 2002; 62: 3691-3697.
- D’Amato R, Lin CM, Flynn E, et al. 2- Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerisation by interacting at the colchicine site. Proc Natl Acad Sci USA 1994; 91: 3964-3968.
- Kamath K, Okouneva T, Larson G, et al. 2- Methoxyestradiol suppresses microtubule dynamics and arrests mitosis without depolymerising microtubules. Mol Cancer Ther 2006; 5: 2225-2233.
- Dahut WL, Lakani NJ, Gulley JL, et al. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic agent, in patients with solid tumors. Cancer Biol Ther 2006; 5: 271-280.
- Sweeney C, Liu G, Yiannoutsos C, et al. A Phase II Multicenter Randomized Double-Blind Safety Trial Assessing the Pharmacokinetics Pharmacodynamics and Efficacy of Oral 2-Methoxyestradiol Capsules in Hormone- Refractory Prostate Cancer. Clin Cancer Res 2005; 11: 6625-6633.
- James J, Murry DJ, Treston AM, et al. Phase I Safety, Pharmacokinetic and Pharmacodynamic Studies of 2- Methoxyestradiol Alone or in Combination with Docetaxel in Patients with Locally Recurrent or Metastatic Breast Cancer. Invest New Drugs 2006; 25: 41-48.
- Tevaarwerk AJ, Holen KD, Alberti DB, et al. Phase I trial of 2-methoxyestradiol NanoCrystal dispersion in advanced solid malignancies, Clin. Cancer Res 2009; 15: 1460-1465.
- Newman SP, Ireson CR, Tutill HJ, et al. The Role of 17B-Hydroxysteroid Dehydrogenases in Modulating the Activity of 2-Methoxyestradiol in Breast Cancer Cells. Cancer Res 2006; 66: 324-330.
- Sano T, Hirasawa G, Takeyama J, et al. 17 beta- Hydroxysteroid dehydrogenase type 2 expression and enzyme activity in the human gastrointestinal tract. Clinical Sci 2001; 101: 485-491.
- Purohit A, Woo LWL, Chander SK, et al. Non-steroidal and steroidal sulfamates: new drugs for cancer therapy. Mol Cell Endocrinol 2001; 171: 129-135.
- Harrison MR, Hahn NM, Pili R, et al. A phase II study of 2-methoxyestradiol (2ME2) NanoCrystal (R) dispersion (NCD) in patients with taxane-refractory, metastatic castrate-resistant prostate cancer (CRPC). Invest New Drugs 2010; 29: 1465-1474.
- Raobaikady B, Purohit A, Chander SK, et al. Inhibition of MCF-7 breast cancer cell proliferation and in vivo steroidsulphatase activity by 2-methoxyoestradiol-bissulphamate. J Steroid Biochem Mol Biol 2003; 84: 351-358.
- Newman SP, Foster PA, Stengel C, et al. STX140 is efficacious in vitro and in vivo in taxane-resistant breast carcinoma cells. Clin Cancer Res 2008; 14: 597- 606.
- Visagie MH, Joubert AM. The in vitro effects of 2- methoxyestradiol-bis-sulphamate on cell numbers, membrane integrity and cell morphology, and the possible induction of apoptosis and autophagy in a nontumorigenic breast epithelial cell line. Cell Mol Biol 2010; 15: 564-581.
- Vorster CJ, Joubert AM. In vitro effects of 2- methoxyestradiol-bis-sulphamate on cell numbers, morphology and cell cycle dynamics in the MCF-7 breast adenocarcinoma cell line. Biocell 2010; 34: 71- 79.
- Day JM, Foster PA, Tutill HJ, et al. BCRP expression does not result in resistance to STX140 in vivo, despite the increased expression of BCRP in A2780 cells in vitro after long-term STX140 exposure. Br J Cancer 2009; 100: 476-486.
- Raobaikady B, Reed MJ, Leese MP. Inhibition of MDA-MB-231 cell cycle progression and cell proliferation by C-2-substituted oestradiol mono- and bis-3- O-sulphamates. Int J Cancer 2005; 117: 150-159.
- Suzuki RN, Newman SP, Purohit A, et al. Growth inhibition of multi-drug-resistant breast cancer cells by 2- methoxyoestradiol-bis-sulphamate and 2- ethyloestradiol-bis-sulphamate. J Steroid Biochem Mol Biol 2003; 84: 269-278.
- Visagie MH, Joubert AM. 2-Methoxyestradiol-bissulfamate induces apoptosis and autophagy in a tumorigenic breast epithelial cell line. Mol Cell Biochem 2011; 375: 343-352.
- Mqoco T, Marais S, Joubert A. Influence of estradiol analogue on cell growth, morphology and death in esophageal carcinoma cells. Biocell 2010; 34: 113-120.
- Krysko DV, Vanden Berghe T, D’Herde K. Apoptosis and necrosis: Detection, discrimination and phagocytosis. Methods 2008; 44: 205-221.
- Warenius HM, Kilburn JD, Essex JW, et al. Selective anticancer activity of a hexapeptide with sequence homology to a non-kinase domain of cyclin dependent kinase. Mol Cancer 2011; 10: 72.
- Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol 2004; 36: 2491-2502.
- Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol 2004; 14(2): 70-77.
- Levine B, Yuan J. Autophagy in cell death: an innocent convict?. J Clin Invest 2005; 115: 2679-2688.
- Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 2005; 12: 1509-1518.
- Mizushima N. Autophagy: process and function. Genes Dev 2007; 21: 2861-2873.
- Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature 2008; 451(7182): 1069-1075.
- Klionsky DJ, Emr SD. Autophagy as a Regulated Pathway of Cellular Degradation. Science 2000; 290: 1717-1721.
- Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ 2005; 12: 1528-1534.
- Lorin S, Borges A, Dos Santos LR, et al. c-Jun NH2- Terminal Kinase Activation Is Essential for DRAMDependent Induction of Autophagy and Apoptosis in 2- Methoxyestradiol-Treated Ewing Sarcoma Cells. Cancer Res 2009; 69(17): 6924-6931.
- Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): Implications for Cancer Progression and Treatment. Ant Red Signaling 2009; 11(4): 777-790.
- Crighton D, Wilkinson S, O'Prey J, et al. DRAM, a p53-Induced Modulator of Autophagy, Is Critical
- for Apoptosis. Cell 2006; 126: 121-134.
- Yokoyama T, Miyazawa K, Naito M, et al. Vitamin K2 induces autophagy and apoptosis simultaneously in leukemia cells. Autophagy 2008; 4: 629-640.
- Eisenberg-Lerner A, Bialik S, Simon H-U, et al. Life and death partners: apoptosis, autophagy and the crosstalk between them. Cell Death Diff 2009; 16: 966-975.
- Maiuri MC, Criollo A, Kroemer G. Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J 2010; 29: 515-516.
- Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J 2011; 278: 403-413.
- Fimia GM, Piacentini M. Regulation of autophagy in mammals and its interplay with apoptosis. Cell Mol Life Sci 2010; 67: 1581-1588.