ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2011) Volume 22, Issue 1
Xolair: A New Monoclonal Drug Anti-IgE Antibody for the Treatment of Allergic Asthma
Department of Pharmaceutical Science, University of Salerno
1Respiratory and Physiopatology Department, Cava de’ Tirreni Hospital, Italy
2S. Maria Incoronata Olmo Hospital, Italy
- *Corresponding Author:
- Anna Capasso
Department of Pharmaceutical Science
University of Salerno
Italy
Accepted date: October 20 2010
Xolair is a monoclonal antibody that binds the Cε3 domain of IgEs, inducing a conforma-tional change of the immunoglobulin, a concealment of FcεRI and FcεRII receptors binding sites, thus precluding binding by IgEs and therefore stopping the release of inflammation mediators. Xolair is indicated as add-on therapy to improve/control asthma in adult and adolescent patients (12 years of age and above) suffering from severe persistent allergic asthma. The aim of our work was to evaluate the Xolair efficacy in the asthma treatment. Six patients (4 men and 2 women aged 30 to 60 years) were selected for the treatment with Xolair. Previously, they were treated with high doses of long-acting β2-agonists and inhaled corticosteroids, but they were not able to control their illness despite the assumption of such drugs. Xolair was administered for 32 weeks in addition to the traditional asthma therapy. Xolair dosage was calculated according to their weight and their IgE levels (IU/ml). The the-rapeutic response was evaluated according to the GETE (Global Evaluation of Treatment Effectiveness) scale. The persistence of the response was defined, instead, by the number of patients continuing to respond positively to the treatment between the 16th and the 32nd week. 4 of 6 patients concluded the study. Patient number 2, in fact, had a body weight higher than 150 kg, so it was impossible to establish the necessary drug dosage. Patient number 6, instead, still had too high IgE values at his fourth checkup. Patient number 1 was fit in the efficacy level 2 (Good) at the 16th week, while at the 32nd week, he was in the level 1 (Excellent). Patient number 3 was at an efficacy level 1 yet from the 16th week, and that level was confirmed at the 32nd. In patient number 4, as well in that number 1, an increase of treatment efficacy was proved with a passage from level 2 to level 1. Finally, for the patient number 5, the efficacy level was Good both at the 16th and at the 32nd week (level 2). The re-sults obtained were positive for all patients and not only the persistence of a therapeutic re-sponse was confirmed, but in some cases there was an improvement of efficacy. Therefore, we can conclude that Xolair, administrated as an additional therapy, strongly improved the severe persistent IgE-mediated asthma. The anti-IgE Xolair treatment reduces the asthma frequency, also, it improved the patient life quality, by inducing positive effects on symp-toms and pulmonary function.
Keywords
Asthma, allergy, inflammation, Ig-E
Introduction
Xolair is a monoclonal antibody identified through so-matic cell hybridization techniques [1]. First, researchers isolated a murine anti-IgE antibody (MAE11) that pre-vented binding of IgE to FcεRI receptors on basophils and mast cells [2,3]. Later, MAE1 was humanized and now it is a monoclonal antibody having just approximately 5% nonhuman amino-acid residues.
Xolair binds the Cε3 domain of IgEs, thus inducing a con-formational change of the immunoglobulin and provoking a concealment of FcεRI and FcεRII receptors binding sites, thus precluding binding by IgE
Xolair binds only free and not bound human IgEs. The bond of the anti-IgE prevents basophils and mast cells de-granulation, therefore it stops the release of inflammation mediators.
Different studies have shown that Xolair reduces basophil FcεRI receptors expression [4,5]. The drug has got a low immunogenic potential, it forms little and biologically in-active complexes with IgE, it doesn’t fix complement and doesn't provoke anaphylaxis. It is considered as add-on therapy to improve asthma control in adult and adolescent patients (12 years of age and above) suffering from severe persistent allergic asthma, having positive skin test or in vitro reactivity to a perennial aeroallergen, a reduced lung function (FEV1<80%) as well as frequent daytime symp-toms or night-time awakenings and data referring re-peated severe asthma exacerbations, despite a daily as-sumption of high-dose inhaled corticosteroids and a long-acting inhaled beta2-agonist.
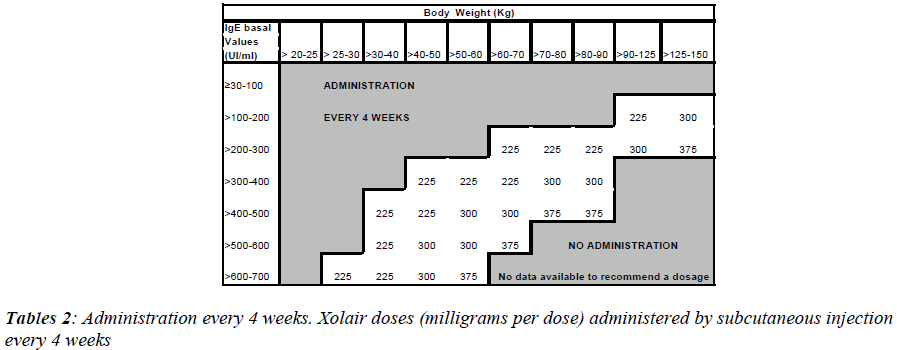
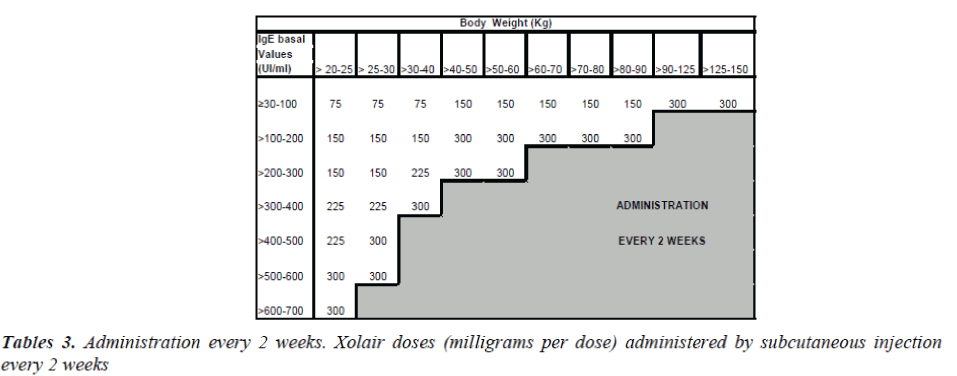
Xolair treatment must be considered only for patients having a clear IgE-mediated asthma. The appropriate dose and the frequency of administration are determined by basal IgE levels (UI/ml), measured before the beginning of treatment and by the body weight (Kg).
Patients with IgE lower than 76 IU/ml are less likely to experience benefits. The dosage varies between 75 and 375 mg of Xolair, to be administered subcutaneously every 2-4 weeks, depending on the above-mentioned pa-rameters. The drug has not to be administered by intrave-nous or intramuscular injection. Subcutaneous injections are practiced in the deltoid region or in the thigh, by a sanitary operator.
16 weeks after the beginning of the therapy with Xolair, patients must be visited by their own physician, in order to verify the effectiveness of the treatment before further injections are practiced. The decision to continue Xolair therapy is based on the observation of a marked im-provement of asthma general management. Doses must be modified in case of relevant variations of body weight.
Data on the use of Xolair in patient over the 65 years of age are few, but there is no evidence that elderly patients need a dosage different from that of younger adult pa-tients. The safety and the effectiveness in pediatric pa-tients under age 12 have not been established, so it is not recommended to use the drug.
Xolair is not suitable for the treatment of acute asthmatic exacerbations, acute bronchospasm or asthmatic state. Besides, it has not been studied in patient with suffering from hyperimmunoglobulin E syndrome or allergic bron-chopulmonary aspergillosis, neither for the prevention of anaphylactic reactions, those provoked by food allergy included. Patients with diabetes mellitus must be in-formed that a 75 mg Xolair dose contains about 54 mg of sucrose.
During clinical studies, the more frequently adverse reac-tions signaled have been local reactions at injection site, including pain, swelling, erythema, itch, headache.
The drug was approved by the FDA in June 2003 for the employment in adult patients and teen-agers (> 12 years) suffering from persistent severe allergic asthma, inade-quately controlled by therapy with inhaled corticosteroids. Its usage was authorized in different countries and re-cently it has been approved from the European Union (July 2005).
In Italy, Xolair is currently in course of studies in more than 30 hospital centers, among them the Respiratory Physiopathology of Cava de' Tirreni Hospital, directed by professor Mario Polverino. This is a multicenter, interna-tional, randomized open trial, for parallel groups, to ap-praise the persistence of response to Xolair treatment and to evaluate the effect of its association to the optimal asthma therapy, compared with the asthma therapy alone. Besides, changes of clinical parameters and patients qual-ity of life were evaluated, so to promote the development of clinical indications for the new drug administration.
Xolair has been administered for 32 weeks in addition to the traditional asthma therapy, to adult patients and teen-agers with severe and persistent allergic asthma, not ade-quately controlled, despite taking regularly inhaled corti-costeroids and long-acting inhaled β2-agonists.
The aim of this work is to study the efficacy of Xolair and its potential therapeutic application.
Methods
Experimental protocol
This work aims to identify treatment guidelines for pa-tients well-responding to treatment with Xolair after 16 weeks as well as to evaluate the persistence of results. The drug was administrated in addition to the classic asthma therapy in patient with moderate to severe persis-tent asthma and whose symptoms were inadequately con-trolled with inhaled corticosteroids.
• The therapeutic response has been evaluated accord-ing to the GETE (Global Evaluation of Treatment Effectiveness) scale, determined according to the number of patients succeeding in a total control of asthma or improving their condition after 16 weeks. The persistence of the answer has been defined, in-stead, by the number of patients continuing to an-swer positively to the treatment between the 16th and the 32nd week and considering the following pa-rameters:
• GETE test carried out at the 16th and the 32nd week.
• Evaluation of the pulmonary function (FEV1) at the 16th and the 32nd week.
• Frequency of relevant and severe asthmatic exacer-bation during the 32nd week of treatment.
• Reduction of oral corticosteroids utilization.
• Asthma symptoms evaluated through the Asthma Control Questionnaire (ACQ) and evaluation of night-time awakening frequency at the 16th and 32nd week.
• Frequency of hospital refuges and visits in emer-gency room or at ambulatory, because of symptoms worsening.
• Evaluation of quality of the life through compilation of AQLQ and EQ-5D questionnaires to the 15th and 31st week.
• Impairment of working activity measured through the WPAI-AA questionnaire (or through the Ado-lescent Productivity Impairment Evaluation in the case of teen-agers) at the 15th and 31st week.
• Evaluation of the best single clinical parameter or the best combination of clinical parameters to estab-lish the response to the treatment with Xolair.
Experimental sketch
The study has been divided in 4 periods:
- Screening period
- run-in period
- open, parallel-group treatment
- open treatment period to allow the access to the drug
Screening period (1 week)
During this period the eligibility of patients to the study has been evaluated. Patients had to suffer from allergic asthma, reduced pulmonary functionality and to have a history of inadequate control of asthma despite therapy with high doses of inhaled corticosteroids (ICS) and long-acting β2-agonists (LABA).
Run-in period (8 weeks)
During the first 4 weeks, asthma therapy has been reval-ued and optimized. Patients were given advices on how to avoid the exposure to allergens, on the monitoring of therapy with theophylline (if applicable) and on tech-niques of inhalation. Throughout the run-in period pa-tients had to compile a diary, reporting daytime and noc-turnal symptoms.
In the last 4 weeks of the run-in period, before randomiza-tion, no dosage adjustment of the asthma therapy has been allowed. Patients had to keep on manifesting an inade-quate control of asthma despite the optimal therapy based on high doses of corticosteroids (> 1000 μg be-clomethasone dipropionate or equivalent) in association to LABA.
The run-in period could be prolonged in the case of asthma exacerbation during the last 4 weeks. In this case, after the resolution of the exacerbation, the patient had to receive for 4 weeks the optimal established manteinance therapy, before being randomized. The run-in period has not lasted more than 12 weeks.
Open, parallel-groups treatment period (32 weeks)
Patients have been randomized in a ratio of 2 to 1 to Xo-lair treatment in association with the optimal asthma ther-apy or with the only optimal asthma therapy for 32 weeks. During this period, asthma therapy has been periodically re-evalued and eventually modified.
Open treatment period to allow the access to the medicine
After the 32 weeks, patients could continue Xolair treat-ment on the basis of an open extension protocol, assuring the access to the drug up to 3 months after its marketing or at the latest for 24 months after the completion of the study.
Patients examination
Adult and young patients (> 12 years) with persistent se-vere allergic asthma and a documented evidence of in-adequate control of the asthmatic sympthomatology de-spite the treatment with high doses of ICS and LABA.
Inclusion criteria
• Patient of both genders, aged ≥ 12 and ≤ 75 years and weighing ≥ 20 and ≤ 150 kg, giving their written in-formed consent.
• Total serum IgE basal levels ≥ 30 and ≤ 700 IU/ml
• Diagnosis of allergic asthma according to the ATS (American Thoracic Society) criteria, from 1 year at least and anamnesis consistent with the clinical char-acteristics of the steps 3 or 4 of GINA guidelines
• positive skin test (wheals diameter ≥ 3 mm), dated no more than 2 years before the visit 1, to at least a per-ennial allergen, to which the patient will regularly be exposed for the whole duration of the study. In pa-tients showing uncertain skin test, a RAST can be ex-ecuted.
• Patients with total IgE ≤ 76 IUs/ml need a positive RAST to be included.
• Increase ≥ 12% of the FEV1 compared to the basal value in the 30 minutes following the inhalation of 2-4 puffs of salbutamol (2-4 for 100 μg) or salbutamol nebulized up to 5 mg (or another β2-agonist) docu-mented during the last 2 years, the screening or run-in periods, or before randomization
• FEV1 ≥ 40 e ≤ 80% of the theoretical value for the pa-tient (remarkable at least 6 hours after the inhalation of a short-acting β2-agonist or 12 hours after the in-halation of long-acting β2 adrenergic) to the ran-domization
• High doses corticosteroids (≥ 800 μg BDP or similar) plus a LABA (assumed continuously) treatment at least for 3 months before the screening and > 1000 μg BDP + LABA at least for 4 weeks during the run-in period and from the randomization
• History of at least 2 severe asthmatic exacerbation in the last 3 years, at least 1 of them in the 12 months preceding the screening, requiring a treatment with systemic corticosteroids additionally to the inhaled corticosteroids and LABA
• Evidence of a non adequate control of the asthmatic symptomatology at the screening (on the basis of the anamnesis) and during the 4 weeks preceding ran-domization. Symptoms are so-called not controlled when one or more of the following criteria are pre-sent:
• Nocturnal awakenings for asthmatic (on average more than once a week) symptomatology;
• Limitation of normal daily activities, the physical ex-ercise included, because of asthmatic symptoms;
• Presence of symptoms during the day (more than 2 days a week) requiring an appeal to the rescue medi-cation;
• Regular employment of rescue medication (≥ 3 times a week);
• PEFR variability (difference between evening and morning values) ≥ 20% measured before the assump-tion of a bronchodilator, for 2 consecutive days dur-ing the 2 weeks preceding weeks randomization.
Main exclusion criteria
• Pregnancy and nursing.
• Patients starting a pregnancy during the study must suspend the treatment and being monitored until the birth of their child. Women in fertile age, not using a reliable contraceptive method (hormones, double barrier). Women who have had a hysterectomy or a surgical sterilization and women in menopause can take part.
• Use of systemic corticosteroids for conditions different from allergic asthma in the 4 weeks pre-ceding the visit 1 or during the study
• Impossibility to do a washout from short- or me-dium-acting antihistaminic before the skin tests of the visit 1. Their utilization is allowed during the study but the washout is required before the skin tests:
• Short-acting (es. chlorpheniramine, prometazina, diphenhydramine) at least 3 days before the visit 1
• Medium-acting (es. loratadine, cetirizine, fexofe-nadine) 5 days before the visit 1
• Concomitant or expected use of beta-blockers during the study
• Use of metotrexate, gold salts, cyclosporine or troleandomycin in the 3 months before the visit 1
• Use of desensitizing therapy maintenance dos-ages, not stable from at least 3 months before the visit of screening (visit 1)
• Use of additional therapies for asthma during the last 4 weeks of run-in
• Use of oral or inhaled anticholinergics within 24h from the visit 1
• Treatment of an exacerbation episode of the asthmatic symptomatology during the 4 weeks preceding weeks randomization
• History of anaphylactic reactions to foods or drugs. History of allergy to antibiotics. Patients can be included if these antibiotics will be avoided during the study. Even in case of aspirin- or other NSAID-related asthma, patients can be included only of these drugs will be avoided.
• History of smoke (> 10 pack-years).
• Active Pneumopathy different from asthma (COPD, chronic bronchitis).
• Increased IgE levels, provoked by conditions dif-ferent from atopy (es. Viral infections, iper-IgE syndrome, Wiskott-Aldrich syndrome or bron-chopulmonary allergic aspergillosis).
• Clinically relevant conditions (neoplasies, infec-tions, hematological, kidney, liver, endocrine, ga-strointestinal, cardiovascular diseases) in the 3 months preceding the study.
• Acute sinusitis or respiratory infections in the month before the visit 1.
• Relevant clinical ECG anomalies in the month preceding the visit 1.
• Relevant clinical alterations laboratory tests found during the visit 1.
• Previous involvement in a clinical study with Xo-lair or patients that had already used the medi-cine.
• Known hypersensitivity to any component, in-cluded the excipients of Xolair
• Alcohol and drugs abuse
Treatment with Xolair
At the end of the run-in, patients have been randomized through IVRS (Interactive Voice Randomization System) to receive the treatment with Xolair added to optimal asthma therapy or with the only optimal asthma therapy.
The drug was in lyophilized form and had to be dissolved in purified water for injections, at the moment of injec-tion. The reconstituted solution showed a weak yellow low coloration and it was slightly viscous. The subcuta-neous administration lasted 5-10 seconds and it was re-peated every 2 or 4 weeks for 32 weeks.
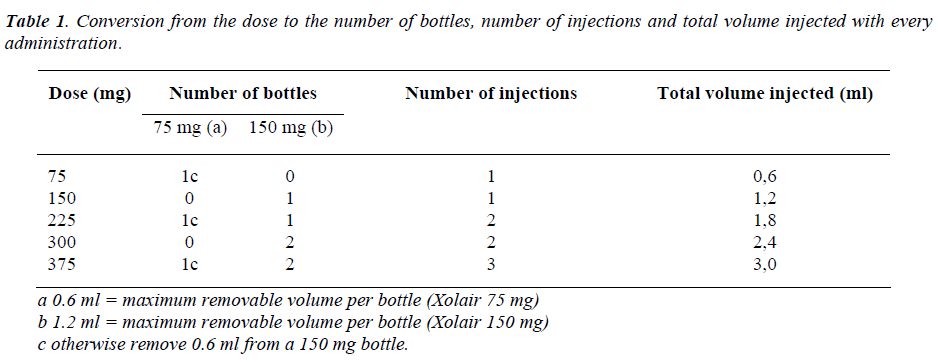
Every phial of Xolair contains 150 mg of active sub-stance, therefore the number of injections per administration was calculated from the total dose and it varied from 1 to 3 injections.
To determine the correct dose and the number of bottles to inject, one has to refer the table 1, while tables 2 and 3 are necessary for every 4 or 2 weeks administrations. Xolair is in monodose phials, it doesn't contain preserva-tive agents and after reconstitution the preparation must be used within 8 hours.
Results
Patients' evaluation in treatment
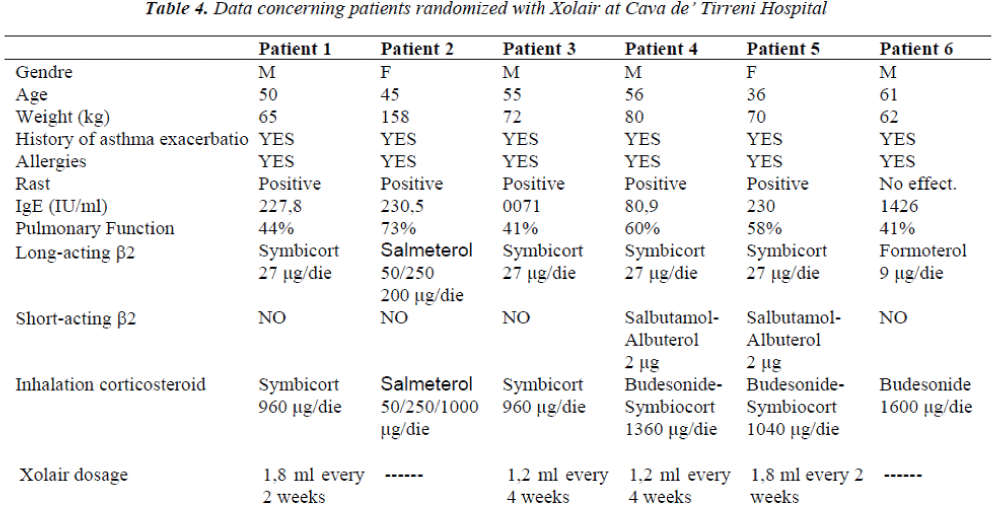
6 patients were selected for the treatment with Xolair in the Department of Respiratory Physiopathology of Cava de’ Tirreni Hospital (Table 4). They were individuals suffering from persistent serious allergic asthma, in ther-apy with high doses of long-acting β2-agonists and in-haled corticosteroids, incapable to control their illness de-spite the assumption of such drugs.
They were patients from 30 to 60 years of age, 4 men and 2 women. They had all undergone to episodes of more or less severe asthmatic exacerbation during the two years preceding the visit of screening, that obliging them to admit to hospital for quite long periods. Many patients were ex-smokers with a history of documented allergy to various types of allergens.
Patients 1,2 and 5 had very similar IgE levels (227-230 IUs / ml) but different percentage of pulmonary function-ality. Patient 3 had a low (71 IUs / ml) IgE level, to the limit of required levels to be included in the study. The RAST test of this patient took, underlined positive results to the dermatophagoides of cat hair, so it was possible to include him in the study.
Patient 6, instead, showed a very high IgE value (1426 IUs / ml) related to other pathologies, and for this reason he was excluded from the treatment with Xolair. Patient 4, finally, had an IgE level equal to 80,9 IUs/ml.
The percentage of respiratory functionality was within the limits of inclusion criteria for all the patients. Patients showed symptoms putting them to the steps 3 or 4 in the classification chart of asthma severity.
All patients signed an informed consent to certify their voluntary share and aware to the study. For patients 2 and 5, besides normal controls, some tests of pregnancy in various phases of the study were performed.
Until the end of run-in period (8 weeks) patients noted on a diary their upper flow values, measured in the morning and in the evening, through a peak gauge provided by the hospital itself. During this period, patients were asked to respond to several questionnaires dealing with their health state and with the influence of asthma on their daily and working life. After an 8 week-study period, patient un-derwent 4 visits, each one every 2 weeks. In these occa-sions the medical staff monitored their vital parameters (pressure, body temperature, pulse, breath frequency), their correct diary compilation with flow peaks and noted any possible variation in therapeutic scheme.
After this control period patients were randomized with the drug. But just 4 of 6 patients concluded the study. Pa-tient number 2, in fact, had a body weight higher than 150 kg, so it was impossible to establish the necessary drug dosage. Patient number 6, instead, still had too high IgE values at his fourth checkup. Xolair dosage administrated to each patient was calculated according to their weight and their IgE levels (IU/ml), as shown in Tables 3 and 4. During Xolair therapy medical staff monitored patients’ vital parameters, spirometric examinations took place in order to evaluate FEV1 and hematochemical exams al-lowed to assess the pulmonary inflammation degree. Pa-tients responded to several questionnaires to evaluate their asthma symptoms; besides, schedules concerning cases of exacerbation or hospitalization were compiled.
Data analysis
The efficacy of Xolair therapy was investigated on the basis of a so defined GETE evaluation scale:
1. Excellent: total control of asthma symptoms
2. Good: pronounced improvement of symptoms
3. Moderate: distinguishable but limited improve-ment of symptoms
4. Poor: no evidence for improvement
5. Worsening
Results were positive for all patients: the persistence of a therapeutic response was not only confirmed, but in some cases there was an improvement of efficacy. Patient num-ber 1, in particular, was fit in the efficacy level 2 (Good) at the 16th week, while at the 32nd week, he was in the level 1 (Excellent). Patient number 3 was at an efficacy level 1 yet from the 16th week, and that level was con-firmed at the 32nd. In patient number 4, as well as in that number 1, an increase of treatment efficacy was proved with a passage from level 2 to level 1. Finally, for the pa-tient number 5, the efficacy level was Good both at the 16th and at the 32nd week (level 2).
From the analysis of questionnaires proposed to patients during the different phases of the study, a considerable improvement of symptoms and a reduction or disappear-ance of night awakenings and daytime asthma symptoms were noticed. Questionnaires proposed were compiled at the hospital or by phone:
• AQL (Asthma Quality of Life) at weeks 15 and 31;
• EQ-5D (Euro Quality of Life-5 Dimension ques-tionnaire) at the screening, at the end of run-in, at weeks 15 and 31;
• WPAI-AA (Work Productivity and Activity Im-pairment or Adolescent Productivity Impairment Evaluation) at the screening, at the end of run-in, at weeks 15 and 31;
• ACQ (Asthma Control Questionnaire) the screening, at the end of run-in, at the 16th and 32nd weeks.
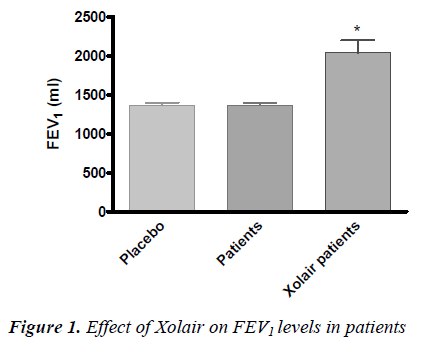
• The level of forced expiratory volume in 1 sec-ond (FEV1) and so the percentage of respiratory function was better in patients treated with Xolair compared to placebo and to patients before treatment (P<0.05) (Fig. 1).
During treatment, there were not exacerbation phenom-ena, nor visits at primary care or admissions.
ICS and LABA anti-asthma therapy did not undergo rele-vant variations in patients.
During the study, patients showed some troubles: phary-ngitis, catarrhal sinusitis, headache, laryngitis. However, no correlation was found between these diseases and Xolair administration, so, no schedule of pharmacovigi-lance was transmitted during this study.
Discussion
Asthma is a very common disease, affecting approxima tively 3-5% of the general population. Since 1970 fre-quency and severity of this condition raised considerably in many countries, involving adults and children without exception [6-8].
About 2/3 of asthamatic forms are allergic forms [9] and there is a strong relationship between allergic asthma de-velopment and IgE levels [4].
Treatment for this disease is based on corticosteroid ad-ministration in order to limit the inflammatory process and β2-adrenergic administration, to contrast broncocon-striction. Patients more seriously ill usually need high doses of such drugs, but they do not always succeed in properly controlling symptoms.
People suffering from severe persistent allergic asthma have symptoms and severe exacerbation despite therapy; they have a heavily conditioned quality of life, tolerate important family and social costs and they affect asthma health costs for over 50% [9,10].
A new pharmacological approach to treat allergic asthma has been recently proposed, that is the use of anti-IgE. Xolair, an anti-IgE, is a monoclonal antibody obtained by genetic engineering methods, which is able to bind IgEs and to inhibit type I hypersensitivity reactions. Its chemi-cal structure, physical properties and binding capability are very similar to an IgG1.
Xolair, whose active ingredient is Omalizumab, is one of the first anti-IgE drugs used in asthmatic patients. This molecule was discovered in 1993 [11], and approved in the USA in 2003 and in the EU in 2005 [11].
Xolair is advisable for the treatment of patients suffering from severe persistent allergic asthma, not having good results from the only optimal anti-asthmatic therapy. The drug is actually in phase II and III clinical trials in several allergic diseases.
The study 1 implicated 419 severe allergic asthmatic pa-tients, aged 12 to 79 years, showing a reduced pulmonary functionality (FEV1 40-80%) and a poor control of their asthmatic symptomatology. Patients had had multiple asthmatic exacerbations over the course of the year before the study and were in therapy with high doses of ICS and LABA. Xolair treatment in those patients reduced the asthmatic exacerbation frequency of 19%. A reduction in emergency visits related to asthma and improvements in the doctor’s global evaluation on the efficacy of treatment were noticed [12].
The study 2 evaluated the efficacy and the safety of Xolair in a population of 312 patients with the same fea-tures of that in the study 1. The treatment with the drug in this open study showed a 61% reduction in the fre-quency of clinically relevant asthmatic exacerbations, compared to the only anti-asthma therapy in course [13].
Other four studies lasting 28 or 52 weeks were conducted on1722 patients. In these situations, the efficacy and safety of Xolair therapy were evaluated in patients with severe persistent asthma. Studies 3 and 5 evaluated asthma exacerbations as primary objective, while the study 6 evaluated the reduction of inhaled corticosteroids [14].
In the studies 3, 4 and 5 patients treated with Xolair pre-sented, respectively, a 37,5%, 40,3% and 57,6% reduction in the frequency of asthmatic exacerbations, compared to placebo. In the study 6 more severe asthmatic patients treated with Xolair succeeded in reducing Fluticasone dosage to ≤ 500 μg/die, without loosing the control of asthma (60,3%) compared to placebo group (45,8%). Quality of life was measured using the Juniper Asthma-related Quality of life.
In all 6 studies there was a statistically significant im-provement of quality of life for Xolair patients compared to placebo or to control group. Besides, a significantly higher percentage of patients treated with Xolair reached a clear improvement or a total control of asthma com-pared to patients treated with placebo.
Pulmonary Physiopathology Department of Cava de’ Tir-reni Hospital was involved the Xolair study by selecting 6 patients for the treatment with Xolair. Only 4 of these pa-tients concluded the study and actually they are going on using the drug in association to the antiasthmatic therapy. The aim of this study was to evaluate the drug efficacy on these patients and to compare results with those already existing in literature.
Patients treated at Cava de’ Tirreni hospital showed a real improvement of their quality of life, in terms of reduction of asthma symptoms.
The evaluation of the treatment efficacy by GETE test was positive for all patients, both at the 16th and at the 32nd week. Besides, there was a significant FEV1 im-provement (P<0,05) compared to patients before Xolair treatment and to placebo.
On the basis of results and of data of literature, we can conclude that Xolair, administrated as an additional ther-apy, decidedly improves tha management of severe per-sistent IgE-mediated asthma. The usage of anti-IgE re-duces the frequency of exacerbation, admission and non-expected visits, improves patient quality life, has positive effects on symptoms and pulmonary function [15].
GINA guidelines recommend Xolair in the pharmacologi-cal treatment of patients with severe asthma; the Scien-tific Counsil of this research organization has indeed evaluated the quality of evidences produced by the drug, founding them at level ‘A’ (the highest).
At this moment Xolair is the only recommended treat-ment with a so high score referred to scientific evidences.
References
- Breedveld FC. Therapeutic monoclonal antibodies. Lan- cet 2000; 355:735-740.
- Hook WA, Zinsser FU, Berenstein EH, Siraganian RP. Monoclonal antibodies defining epitopes on human IgE. Mol Immunol. 1991; 28: 631-639.
- Presta L, Shields R, O’Connel L, et al. The binding site on human immunoglobulin E for its high affinity recep- tor. J Biol. Chem. 1994; 269: 26368-26373.
- Malveaux FJ, Conroy MC, Adkinson NF, Lichtenstein LM. IgE receptors on human basophils. Relationship to serum IgE concentration. J Clin Invest. 1978; 62: 176-181.
- MacGlashan DW, Bochner BS, Adelman DC, et al. Down- regulation of FcepsilonRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997; 158: 1438-1445.
- Skjonsberg OH, Clenchaas J, Leegaard J, et al. Preva- lence of bronchial asthma in schoolchildren in Oslo, Norway – comparison of data obtained in 1993 and 1981. Allergy 1995; 50: 806-810.
- Tirimanna PRS, van Schayck CP, den Otter JJ, et al. Prevalence of asthma and COPD in general practice in 1992: has it changed since 1977? Br J Gen Pract 1996; 46: 277–281.
- Novak N, and Bieber T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 2003; 112: 252-262.
- Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J 2002; 19: 61–67.
- Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, and Jardieu PM. Humanization of an anti-body directed against IgE. J. Immunol. 1993; 151: 2623-2632.
- Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, Beeh KM, Ramos S, Canonica GW, Hedge- cock S, Fox H, Blogg M, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): IN- NOVATE. Allergy 2005; 60: 309-316.
- Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly con- trolled (moderate-to-severe) allergic asthma. Allergy 2004; 59: 701-708.
- Vignola AM, Humbert M, Bousquet J, Boulet LP, Hedgecock S, Blogg M, Fox H, and Surrey K. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy 2004; 59:709-717
- Busse WW. Anti-immunoglobulin E (omalizumab) therapy in allergic asthma. Am. J Respir Crit Care Med 2001; 164: S12-S17.
- Soler M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, Thirlwell J, Gupta N, Della Cioppa G. The anti-IgE an-tibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001; 18: 254-261.
- Holgate ST, Djukanovic R, Casale T, and Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: An update on anti-inflammatory activ- ity and clinical efficacy. Clin Exp Allergy 2004; 35: 408-416.