ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 3
Vitamin E TPGS based liposomal delivery of doxorubicin in osteosarcoma cancer cells
1Department of Bone and Soft Tumor, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou 450008, China
2Department of Respiratory, Children’s Hospital of Zhengzhou City, Henan, Zhengzhou 450053, China
3Department of Orthopedics, the First Affiliated Hospital of Zhengzhou University, Zhengzhou University Zhengzhou 450000, Henan, China
- *Corresponding Author:
- Jia-Qiang Wang
Department of Bone and Soft Tumor
Affiliated Cancer Hospital of Zhengzhou University
Henan Cancer Hospital
Zhengzhou, China
Accepted on August 30, 2016
This research work has been carried out in order to explore the role of Vitamin E TPGS in osteosarcoma (OS). We have prepared TPGS grafted liposome’s loaded with Doxorubicin (TPGS-Lip-DOX) and explored its effect on OS cells. The prepared TPGS liposomes have been characterized for its size, charge, percentage entrapment efficiency. The TPGS-Lip-DOX showed a high entrapment efficiency of 71.5 ± 3.9% with 225 ± 15.6 nm size with homogeneous particles dispersion of PDI, 0.034 ± 0.008. TPGSLip- DOX has showed a sustained release pattern. The cytotoxicity tests exposed that the non-toxicity of control liposome’s and TPGS-Lip-DOX showed concentration dependent toxicity. It was evident that TPGS exerted increased oxidative effect on MG63 cells and TPGS-Lip-DOX facilitates highly lethal effect on MG63 cells and causes apoptosis. The results conclude that the role of TPGS is synergistic as an effective delivery system for cancer chemotherapy
Keywords
Vitamin E TPGS, Osteosarcoma, Liposome.
Introduction
Osteosarcoma (OS) is sort of cancerous tumor originating in bones, which is ubiquitous in teen age and adults of age around 25 years resulting in immature bone and even death in this age group. It is generally originates at the end of long bones, proximal tibia region and distal femur migrating to other bones [1,2]. There are some reports which suggests that OS arises as a subsequent malignant neoplasm as an out-come of prior cancer therapy or genetic predisposition including Rothmund- Thomson syndrome, Bloom syndrome and Li-Fraumeni syndrome [3,4]. Current therapeutic approaches are restricted in their efficiency and have poor prognosis after metastases has occurred in OS and it is difficult to manage with established drug delivery system. The conditions are worse after the chemo-resistance reports in OS patients in a huge section. In this situation, the combination of two or more anti-cancer agents have now been exploited as an effective chemotherapy for OS given i.v. or orally, though their long-term toxic effects cannot be over looked [5,6]. Vitamin E has potential health benefits [7], it is fat soluble and biologically active possessing antioxidant activity and has also been reported to possess preventive effect for cardiovascular disease [8] and carcinogenesis [9], which leads in developing various delivery systems [10]. Vitamin E analogue D-α-tocopherol polyethylene 2000 succinate (TPGS), biocompatibility, safe and non-toxic FDA approved pharmaceutical adjuvant, has been reported to synergise the effect of chemotherapeutic agents by inducing apoptosis [11]. TPGS-coated solid lipid nanoparticles have recently been demonstrated for prolonged systemic circulation and passive brain targeting [12].
The mechanism of TPGS for synergistic effect of chemotherapeutic agents such as curcumin, and doxorubicin (DOX), is inhibition of P-glycoprotein (P-gp), which is an ATP-dependent drug efflux-pump, and thus promotes their cytotoxicity to resistant cancer cells [13,14]. TPGS has several benefits of its own it can be used for control and sustain release of several drugs, the PEG chain of it causes steric hindrance of pancreatic enzymes and stabilizes the liposome’s [15,16]. TPGS has also been used as a solubilizer, emulsifier and stabilizer in various formulations. It enhances the entrapment of drug in nano-delivery systems and it has high uptake in cells in-vitro and with high effects in-vivo. TPGS has also been reported to possess synergistic property to overcome drug resistance in tumor treatment [17]. DOX, an anthracycline antibiotic [18], has been widely used as a chemotherapeutic agent, but due to the development of MDR cancer cells its clinical application are hindered [19]. DOX is known to inhibit deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) synthesis in mammalian cells. DOX also demonstrated inhibition of DNA topoisomerase I [20] and stabilizing topoisomerase II cleavage complexes, leading to DNA strand breaks [21]. DOX has been used in several studies as a single or combined chemotherapeutic drug for leukaemia, breast cancer and lung cancer [22,23]. DOX combined with TPGS may lead to enhanced anticancer activity corresponding to DOX alone.
The chemotherapeutic agents are highly toxic and non-specific to tumor cells and the nano carriers are employed for reducing their toxicity to normal cells and increasing their therapeutic efficacy [24]. Liposomes are now being widely used as a carrier for many chemotherapeutic agents, as it improves therapeutic efficacy of several drugs and the technology of liposomal preparation has been well established in industries. They are even composed of biocompatible non-toxic phospholipids having potential to encapsulate both hydrophilic and hydrophobic drugs [25]. The literature suggests that TPGS coated liposomes have high entrapment (64%), increased cellular uptake and cytotoxicity [26]. We are reporting the liposomes of TPGS encapsulating DOX in order to improve therapeutic outcome of OS treatment through increased DOX concentration in tumor cells and controlled release of DOX.
Material and Methods
D-alpha tocopheryl polyethylene glycol 2000 succinate (TPGS) was purchased from Sigma-Aldrich and 3-(4,5- Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma–Aldrich (St. Louis, USA). Fully hydrogenated soy phosphatidylcholine (HSPC), 1, 2- distearoyl-sn-glycero3-phosphoethanolamine (DSPE) have been purchased from Avanti Polar Lipids, Alabaster, Alabama. Doxorubicin hydrochloride (DOX) has been kindly supplied from Beijing Huafeng United Technology Co., Ltd. (Beijing, China).
Preparation of TPGS grafted DOX loaded liposomes
TPGS grafted liposomes loaded with DOX have been prepared using thin-film hydration method as reported elsewhere. The liposomes were comprised of cholesterol, HSPC, DSPE and TPGS. Briefly TPGS, phospholipids and cholesterol were mixed in different ratios and were dissolved in minimum quantity of a mixture of methanol and chloroform in a ratio of 1:2. Dry lipid film has been obtained by evaporating this mixture in a rotary evaporator which was further hydrated using pre heated (50°C) ammonium sulphate buffer. The hydrated solution was passed through the extruder 5 times using a 0.5 μm polycarbonate membrane followed by 0.2 μm membrane from Millipore. The liposome’s such formed were incubated with a solution of sodium chloride containing DOX. The liposomes were washed and lyophilized to obtain TPGS-Lip- DOX. Liposomes without TPGS have also been prepared by the same method, excluding the addition of TPGS and were used to estimate the effect of TPGS in our studies.
Characterization of TPGS-Lip-DOX
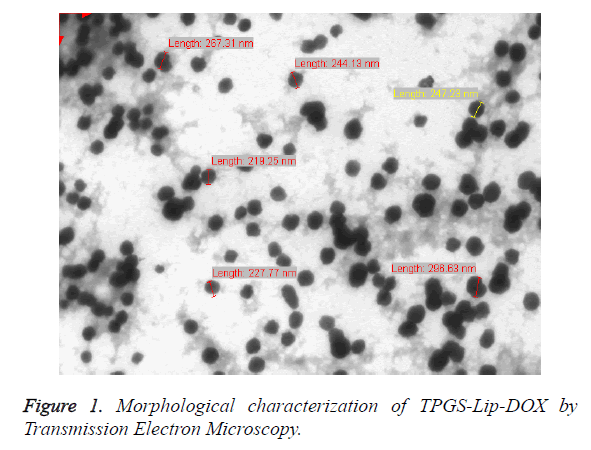
Measurement of size and zeta potential: TPGS-Lip-DOX suspension drop was added to a 200 mesh copper TEM grid. The grid was blotted using 2% (w/v) phosphotungstic acid. The images were captured at 120 kV voltage using 164HR-TEM (TecnaiTMG2 F20, Eindhoven, and The Netherlands). The size and size distributions of TPGS-Lip-DOX were also characterized using a Malvern DLS apparatus. The mean particle sizes, size distributions and zeta potentials were determined using Malvern Zetasizer Nano ZS (Zetasizer 3000 HS; Malvern Instruments Co., UK). Each sample and also the control were measured in triplicate. The charge on the TPGSLip- DOX dispersion i.e. zeta-potential was measured in distilled water at 25°C (Zetasizer 3000 HS; Malvern Instruments Co., UK).
Estimation of percent entrapment efficiency (%EE)
For the estimation of total amount of DOX loaded in TPGS-Lip- DOX, the known amount of TPGS-Lip-DOX was dissolved in 1 mL of organic medium and the amount of DOX was estimated using High performance liquid chromatography having C-18 column and mobile phase as acetonitrile and distilled water having pH of 2.05 using o-phosphoric acid with a flow rate of 1 mL/min and RF-10 AXL Fluorescence detector (Shimadzu) at excitation wavelength of 480 nm and emission wavelength of 550 nm.
In-vitro release
DOX release from TPGS-Lip-DOX has been carried out using Phosphate buffer saline (PBS) of pH 7.4 as a release medium and the amount was estimated using HPLC method reported above. Briefly, TPGS-Lip-DOX, Lip-DOX and free DOX at an equivalent concentration were suspended in 10 ml of PBS in a dialysis bag (cut-off mol. wt. 12 kD) and placed in dissolution apparatus II, which was maintained at 37°Cwith moderate shaking at 100 rpm. After particular time interval, the samples were withdrawn from the medium and medium was replenished every time with equivalent volume of fresh buffer to retain sink condition.
In-vitro studies
Cytotoxicity assay: The cytotoxicity assay was measured by 3-(4, 5-dimethythiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. MG63 OS cells have been seeded at a density of 1 × 106 in a 96-well plate and were incubated for 24 h followed by the treatment of cells with plain DOX, TPGS-Lip-DOX and Lip-DOX at an equivalent concentration of 2.5 μg/mL and 5 μg/mL and the cells were again incubated for 24 h. After incubation, the cells of each well were treated with 100 μl of MTT solution and were again incubated for 4 h. The formazan crystals were dissolved using DMSO and the absorbance of each plate was read at 570 nm using a microplatereader (Thermo-Fisher, USA).
Assay of intracellular reactive oxygen species (ROS)
Generation of ROS in intracellular level was examined using 2′, 7′-Dichlorofluorescin diacetate (DCFDA). DCFDA is a cell-permeable non-fluorescent probe which on oxidation de-esterified intracellularly and forms highly fluorescent 2′, 7′- dichlorofluorescein (DCF). MG63 cells after seeding in a 6- well plate were incubated for one day followed by incubation TPGS-Lip-DOX, Lip-DOX and control liposomes for indicated time points at 37°C with 5% CO2 after which it was the cells have been washed with PBS and incubated in PBS containing 5 μM of DCFDA in dark for half an hour. After which the cells were washed again using PBS and were re-suspended in 500 μl of PBS, and analysed using fluorescence activated cell sorter (FACS). DCF data were recorded at an excitation wavelength of 488 nM and an emission wavelength of 515-540 nM.
In-vitroMG63 cell uptake studies using FACS
TPGS-Lip-DOX and Lip-DOX have been used to estimate the drug internalization in the MG63 cells using FACS. After seeding of MG63 cells in 6 well plate using fresh media, it was incubated for one day subsequentlyMG63 cells were treated with TPGS-Lip-DOX and Lip-DOX and incubated for 4 h. The cells have been washed for thrice using PBS and processed for flow cytometric analysis at an excitation wavelength of 480 nm and an emission wavelength of 550 nm.
Estimation of apoptosis assays
The double stain assay has been performed using Annexin V/PI to carry out apoptosis assay, for which MG63 cells were grown in 12-well plate for one day so that they can adhere at the bottom, followed by the treatment of MG63 cells with TPGS-Lip-DOX and Lip-DOX at an equivalent concentration of 2.5 μg/mL and subsequent incubation for next 24 h. The cells were removed using trypsin and were washed by PBS thrice. The washed cells was collected by centrifugation and were suspended again in PBS, followed by double staining using FITC-annexin V and PI for 20 min and with immediate effect the cells were analysed using FACS.
Statistical analysis
Statistical analysis have been performed by one-way analysis of variance (ANOVA) followed by the Turkey-Kramer, utilizing Graph Pad Instat software (Graph Pad Software Inc., CA, USA).Significance level was P<0.05 for our analysis. Results have been expressed as mean ± SD (n=3).
Results and Discussion
Formulation and physicochemical characterization of TPGS-Lip-DOX and Lip-DOX
The nano-sized particle are now being widely studied and also have been successfully invaded the market for cancer chemotherapy, among which the formulations with PEG on surface as of we have grafted using TPGS in TPGS-Lip-DOX have the potential to evade the macrophages and can selectively accumulate in the cancer tissues. The preparation of TPGS-Lip-DOX has been initiated with the aim of increasing the anticancer activity of DOX in presence of TPGS in order increase the cytotoxic effect of DOX against OS, which has been tested in MG63 cells. The successful liposomal preparation of TPGS-Lip-DOX was achieved and they were physicochemical characterized. The TPGS-Lip-DOX and Lip- DOX showed a high entrapment efficiency of 71.5 ± 3.9% and 63.7 ± 3.1% respectively (Table 1). The high entrapment efficiency in TPGS-Lip-DOX was attributed to the presence of TPGS on the lipid layer and the hydrophilic nature of DOX.
| Formulation | % Entrapment Efficiency | Zeta potential | Average particle size (nm) ± S.D | Poly Dispersity index (PDI) ± S.D |
|---|---|---|---|---|
| TPGS-Lip-DOX | 71.3 ± 3.9 | 33.2 ± 1.4 | 225 ± 15.6 | 0.034 ± 0.008 |
| Lip-DOX | 63.7 ± 3.1 | 29.2 ± 5.7 | 232 ± 12.7 | 0.092 ± 0.005 |
| TPGS-Lip (Control) | - | 24.3 ± 1.2 | 217 ± 11.3 | 0.037 ± 0.009 |
Table 1. Characterization of developed formulations.
The Malvern Zetasizer (DLS) has been used for the estimation of particle size and poly dispersity index (PDI), which revealed the that the average particle size of TPGS-Lip-DOX was 225 ± 15.6 nm with homogeneous particles dispersion (PDI, 0.034 ± 0.008 whereas Lip-DOX have particle size of 232 ± 12.7 nm with PDI of 0.092 ± 0.005). The TPGS-Lip-DOX and Lip- DOX showed a surface charge of 33.2 ± 1.4 mV and 29.2 ± 5.7 respectively and this range is suited for the stability of colloids. The morphology was also established by imaging using TEM as shown in Figure 1. The particle size of TPGS-Lip-DOX was also determined using by TEM and was found to be 200 nm which was in line with the results observed from DLS analysis.
The size obtained using TEM and by DLS was found almost in a range, whereas the slight difference might be due to the fact that DLS profiling is done under aqueous medium but TEM samples are observed as in dry form.
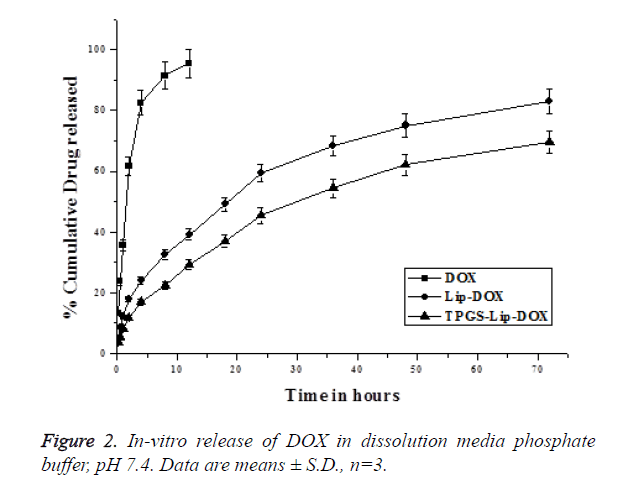
In-vitro release profile
TPGS-Lip-DOX and Lip-DOX, as shown in Figure 2, have not shown burst release initially but plain DOX was very fast in the release medium. The DOX was released in a sustained manner from both the TPGS-Lip-DOX and Lip-DOX and have not shown 100% release within the 72 h where study was terminated. It has to be noted that, the release pattern of DOX from TPGS-Lip-DOX showed more sustained release than DOX from Lip-DOX in which the release was relatively faster. Initially in both the cases the release was below 20% in the till 4 h, slowly the release increases and at 24 h, 46 ± 4.7% of DOX released from TPGS-Lip-DOX comparing to that of 60 ± 3.2% DOX from Lip-DOX. After 72 h, when the study was terminated 82 ± 4.34% of DOX released from the Lip-DOX, whereas only 69 ± 6.4% of DOX has been released from TPGS-Lip-DOX. It has been observed that there was significant difference (p<0.05) in TPGS-Lip-DOX release and Lip-DOX release which might be attributed to the presence of TPGS on the surface of TPGS-Lip-DOX. Nevertheless, a sustained release profile for both was observed which might be of great use in effective cancer treatment.
Cell internalization by cell population (CP) analysis by flow cytometry in MG63 cells
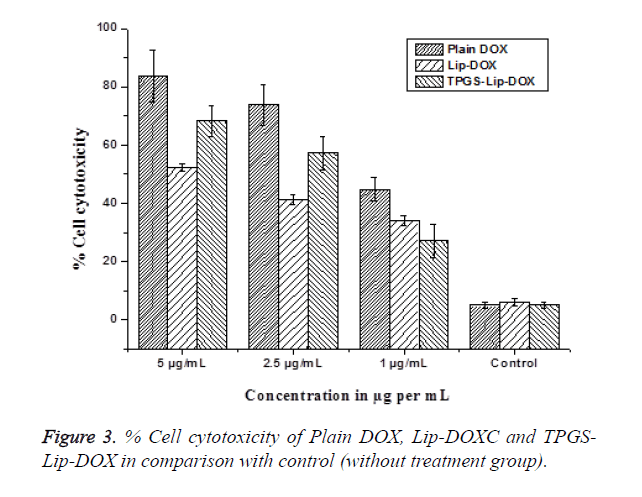
Intracellular uptake in MG63 cells were studied by Flow cytometry analysis using Hoechst dye. This experiment manifests an exclusive pattern of distribution of cells and has direct correlation with the reticence of Hoechst transport and PGP modulation. Since TPGS is a PGP inhibitor its effect on PGP was evaluated and compared by means of flow cytometry. Hoechst dye is used as PGP substrate and Hoechst influx is proportional to the PGP inhibition. Our findings points towards CP of Hoechst dye in cells treated with TPGS-Lip-DOX enhanced up to 6.3 folds. Consequently, the intensity of fluorescence also increased in order TPGS-Lip-DOX>Lip- DOX so increased in the cellular. The biological mimicking components of liposomes are responsible for its recognition as a safe delivery system. The biocompatibility of our TPGS-Lip- DOX and Lip-DOX has also been evaluated in MG63 OS cells (Figure 3). The cytotoxicity tests exposed that the non-toxicity of control liposomes i.e. liposomes without DOX and whereas free DOX, TPGS-Lip-DOX and Lip-DOX showed concentration dependent toxicity.
Among which, free DOX was most toxic than DOX present in the formulation across all the concentration on different; which might be attributed to the easy diffusion of free DOX across the cell membrane and sustain release pattern of DOX from TPGS-Lip-DOX and Lip-DOX after opsonisation in the cells through specific pathways. Importantly, TPGS-Lip-DOX significantly arrested the growth of cancer cells in comparison with the Lip-DOX. The enhanced anticancer effect of TPGS-Lip- DOX might be attributed to the presence TPGS causing significant internalization of TPGS-Lip-DOX inside the cells.
Assay of intracellular reactive oxygen species (ROS)
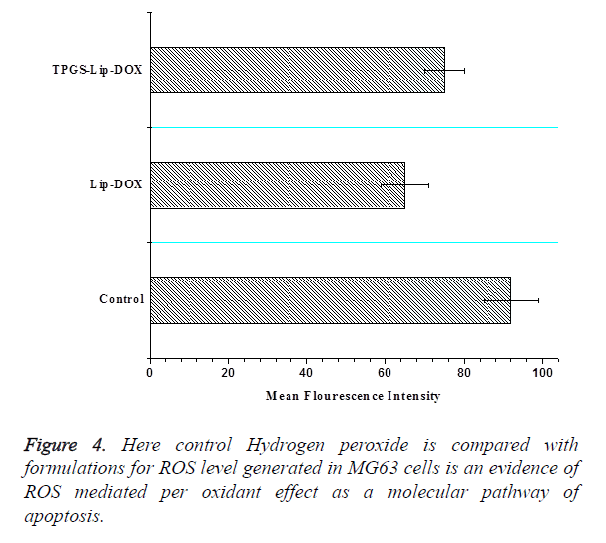
Intracellular ROS is a potential approach for cancer therapies based on raising the ROS level to toxic level for creating the lethal effects in cancer cells. Oxidative stress is triggered by direct ROS accumulation in cancer cells or inhibiting the endogenous antioxidant coordination for selective execution of cancer cells. Our finding emphasized with strong evidences that TPGS exerted increased oxidative effect on MG63 cells and TPGS-Lip-DOX facilitates highly lethal effect on MG63 cells (Figure 4).
In-vitro cellular uptake
For this purpose, MG63 OS cells have been treated with TPGS-Lip-DOX and Lip-DOX followed by incubation at 37°C for 4 h. The results of which indicated specific uptake of TPGS-Lip-DOX and Lip-DOX demonstrating the significant cellular internalization of TPGS-Lip-DOX in comparison with Lip-DOX via endocytosis which clearly indicates the role of TPGS presence in the liposomes causing enhanced DOX concentration, which further reflects the potential of TPGS-Lip- DOX as effective chemotherapeutic agent.
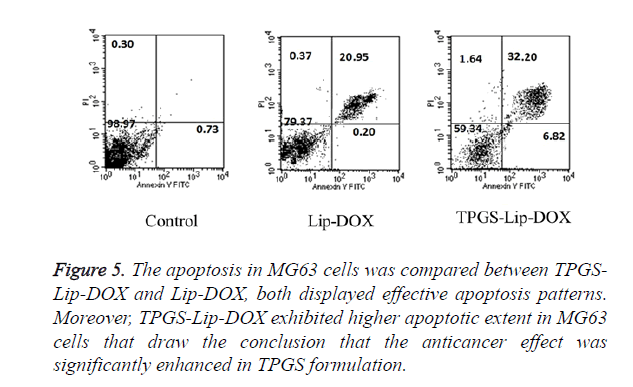
Apoptosis assay
Apoptosis, which has been known for its programmed cell death, has a critical role in tissue homeostasis. The Apoptosis can be detected by the change in cell components such as phosphatidylserine turn over. In our experiment, cell apoptosis in MG63cancer cells was estimated using double dye Annexin V FITC and PI staining. The MG63 cells were incubated with TPGS-Lip-DOX and Lip-DOX. As evident in Figure 5, as expected, TPGS-Lip-DOX induces significant apoptosis in comparison with Lip-DOX in MG63 cells. It was clearly evident in the results the TPGS has remarkably improved the efficacy of DOX and as a consequence it has better chemotherapeutic effect. Even though, apoptosis has also been perceptible in Lip-DOX, advantage of TPGS in TPGS-Lip- DOX can clearly be observed.
Figure 5. The apoptosis in MG63 cells was compared between TPGS-Lip-DOX and Lip-DOX, both displayed effective apoptosis patterns. Moreover, TPGS-Lip-DOX exhibited higher apoptotic extent in MG63 cells that draw the conclusion that the anticancer effect was significantly enhanced in TPGS formulation.
Conclusions and Perspectives
In conclusion, DOX loaded TPGS-Lip-DOX has been successfully prepared and physiochemically characterized. The synergistic effect of the formulation was demonstrated in-vitro using MG63 OS cells. We have clearly proved the synergistic effect of TPGS in our formulation by evaluating in-vitro parameters. The TPGS-Lip-DOX has homogeneous particles size with a pattern of sustained release of DOX. The TPGS-Lip- DOX in comparison with formulation without TPGS (Lip- DOX)potentially inhibit MG63 OS cells when incubated at the same dose of DOX for 24 h. TPGS-Lip-DOX have also shown significant uptake in MG63 cells in comparison with Lip- DOX. TPGS grafting resulted in improved cell uptake and apoptosis, concluding remarkable role of TPGS in improving the efficacy of DOX and opened the possibility of TPGS use in chemotherapy of not only OS but also for other type of cancer.
References
- Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer 2009; 125: 229-234.
- Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev 2006; 32: 423-436.
- Hamre MR, Severson RK, Chuba P, Lucas DR, Thomas RL, Mott MP. Osteosarcoma as a second malignant neoplasm. Radiother Oncol 2002; 65: 153-157.
- Malkin D, Li FP, Strong LC, Fraumeni JF, Nelson CE, Kim DH. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990; 250: 1233-1238.
- Patel SJ, Lynch JW, Johnson T, Carroll RR, Schumacher C, Spanier S. Dose-intense ifosfamide/doxorubicin/cisplatin based chemotherapy for osteosarcoma in adults. Am J Clin Oncol 2002; 25: 489-495.
- Wan J, Zhang X, Liu T. Strategies and developments of immunotherapies in osteosarcoma. Oncol Lett 2016; 11: 511-520.
- Wong RS, Radhakrishnan AK. Tocotrienol research: past into present. Nutr Rev 2012; 70: 483-490.
- Saremi A, Arora R. Vitamin E and cardiovascular disease. Am J Ther 2010; 17.
- Venkateswaran V, Fleshner NE, Sugar LM, Klotz LH. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res 2004; 64: 5891-5896.
- Guo Y, Luo J, Tan S, Otieno BO, Zhang Z. The applications of Vitamin E TPGS in drug delivery. Eur J Pharm Sci 2013; 49: 175-186.
- Mi Y, Liu Y, Feng SS. Formulation of Docetaxel by folic acid-conjugated d-alpha-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS(2k)) micelles for targeted and synergistic chemotherapy. Biomaterials 2011; 32: 4058-4066.
- Vijayakumar MR, Kumari L, Patel KK, Vuddanda PR, Vajanthri KY, Mahto SK. Intravenous administration of trans-resveratrol-loaded TPGS-coated solid lipid nanoparticles for prolonged systemic circulation, passive brain targeting and improved in vitro cytotoxicity against C6 glioma cell lines. RSC Advances 2016; 6: 50336-50348.
- Collnot EM, Baldes C, Wempe MF, Hyatt J, Navarro L, Edgar KJ. Influence of vitamin E TPGS poly(ethylene glycol) chain length on apical efflux transporters in Caco-2 cell monolayers. J Control Release 2006; 111: 35-40.
- Bansal T, Akhtar N, Jaggi M, Khar RK, Talegaonkar S. Novel formulation approaches for optimising delivery of anticancer drugs based on P-glycoprotein modulation. Drug Discov Today 2009; 14: 1067-1074.
- Hsu KH, Carbia BE, Plummer C, Chauhan A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur J Pharm Biopharm 2015; 94: 312-321.
- Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005; 26: 2713-2722.
- Ba-Ya L, Cong W, Xiao-Yan H, Ren-Xi Z, Si-Xue C. Multi-drug loaded vitamin E-TPGS nanoparticles for synergistic drug delivery to overcome drug resistance in tumor treatment. Science Bulletin 2016; 61: 552.
- Dwivedi P, Kansal S, Sharma M, Shukla R, Verma A, Shukla P. Exploiting 4-sulphate N-acetyl galactosamine decorated gelatin nanoparticles for effective targeting to professional phagocytes in vitro and in vivo. J Drug Target 2012 Dec; 20: 883-896.
- Deepa K, Singha S, Panda T. Doxorubicin nanoconjugates. J Nanosci Nanotechnol 2014; 14: 892-904.
- Foglesong PD, Reckord C, Swink S. Doxorubicin inhibits human DNA topoisomerase I. Cancer Chemother Pharmacol 1992; 30: 123-125.
- Capranico G, Kohn KW, Pommier Y. Local sequence requirements for DNA cleavage by mammalian topoisomerase II in the presence of doxorubicin. Nucleic Acids Res 1990; 18: 6611-6619.
- Duan J, Mansour HM, Zhang Y, Deng X, Chen Y, Wang J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm 2012; 426: 193-201.
- Joseph MM, Aravind SR, George SK, Pillai RK, Mini S, Sreelekha TT. Co-encapsulation of Doxorubicin with galactoxyloglucan nanoparticles for intracellular tumor-targeted delivery in murine ascites and solid tumors. Transl Oncol. 2014; 7: 525-536.
- Ringhieri P, Pannunzio A, Boccarelli A, Morelli G, Coluccia M, Tesauro D. Effect of cisplatin containing liposomes formulated by unsaturated chain-containing lipids on gynecological tumor cells. J Liposome Res 2016; 5: 1-6.
- Fanciullino R, Ciccolini J. Liposome-encapsulated anticancer drugs: still waiting for the magic bullet? Curr Med Chem 2009; 16: 4361-4371.
- Weecharangsan W, Yu B, Liu S, Pang JX, Lee LJ, Marcucci G. Disulfide-linked liposomes: effective delivery vehicle for Bcl-2 antisense oligodeoxyribonucleotide G3139. Anticancer Res 2010; 30: 31-37.