ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 1
Valosin-containing protein is a possible sero-diagnostic marker of psoriatic arthritis
1Department of Dermatology, Kitasato University School of Medicine, 1-15-1 Kitasato Minami-ku, Sagamihara, Kanagawa, Japan
2Department of Applied Tumor Pathology, Kitasato University Graduate School of Medical Sciences, 1-15-1 Kitasato, Minami-ku, Kanagawa, Japan
3Department of Clinical Cancer Prevention-Research, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX77030, USA
- *Corresponding Author:
- Yuichi Sato
Department of Applied Tumor Pathology
Kitasato University Graduate School of Medical Sciences
Kitasato 1-15-1, Minami-ku
Sagamihara, Kanagawa 252-0374, Japan
Accepted on Jun 20, 2016
To identify diagnostic markers of psoriasis vulgaris and psoriatic arthritis, autoantibodies in sera from patients with psoriasis were screened by two-dimensional immunoblotting (2D-IB). We previously found 22 autoantigens each in psoriasis sera. In this study, serum levels of valosin-containing protein (VCP) in patients, one of the identified autoantigens in psoriasis patients, were studied by reverse-phase protein array analysis using sera from patients with psoriasis and healthy controls. Serum levels of VCP were significantly higher in patients with psoriatic arthritis than in controls or patients with psoriasis vulgaris (P<0.05 each). The area under the curve for VCP between patients with psoriatic arthritis and controls or psoriatic vulgaris patients was 0.88 and 0.77, respectively. The serum level of VCP was weakly correlated with the disease activity of psoriasis patients and psoriasis area severity index score. These data suggest that VCP is a novel and differential sero-diagnostic marker of psoriatic arthritis.
Keywords
Sero-diagnosis, Valosin-containing protein, Psoriatic arthritis, Reverse-phase protein array.
Introduction
Psoriasis is a chronic, relapsing, and inflammatory skin disease that affects 2% of Caucasians [1], but 0.4% of the population in Japan. This skin condition is histologically characterized by the abnormal proliferation of keratinocytes and infiltration of immune cells, predominantly T-cells, and dendritic cells, in psoriatic lesions [2]. The biomarkers of psoriasis have been used to measure disease severity, to objectively monitor the treatment response, to find new targets for therapy, and to explain comorbidities in psoriasis patients [3,4]. However, there is still no sufficient disease-specific or diagnostic serum marker of psoriasis vulgaris or psoriatic arthritis. The search for new biomarkers of psoriasis is important for not only diagnostic tools for psoriasis but also to develop new therapy. To discover novel and differential sero-diagnostic markers of psoriasis vulgaris and psoriatic arthritis, we previously identified psoriasis-associated proteins that were recognized by autoantibodies in sera from patients with psoriasis vulgaris or psoriatic arthritis as a first antibody by immunoblotting based on two-dimensional gel electrophoresis. As the results, we identified autoantigens like stress-induced phosphoprotein-1 (STIP1), moesin, and valosin-containing protein (VCP) in sera from psoriasis vulgaris and psoriatic arthritis patients. The levels of moesin and STIP1 were significantly higher in sera from patients with psoriasis vulgaris than in healthy controls. In addition, STIP1 was significantly higher in sera from patients with psoriatic arthritis than in those with psoriasis vulgaris. We previously reported that STIP1 and moesin may be novel and differential sero-diagnostic markers of psoriasis vulgaris and psoriatic arthritis [5]. However, a detailed study of serum VCP protein levels in patients with psoriasis has yet to be conducted. In this study, we evaluated whether the serum VCP protein level was a useful diagnostic marker in patients with psoriasis vulgaris and psoriatic arthritis based on a comparison with healthy controls.
Materials and Methods
Ethics statement
This study was approved by the Ethics Committee of Kitasato University School of Medicine (number B10-93). All patients and healthy controls were approached based on approved ethical guidelines, they agreed to participate in the study, and could refuse entry and discontinue participation at any time. All participants provided written consent.
Sera
We obtained sera from 23 patients with psoriasis vulgaris, 11 patients with psoriatic arthritis, and 11 healthy controls in this study. We recorded the sex, age, and disease duration, as well as conducted an assessment of the disease severity using the psoriasis area and severity index (PASI) and percentage of the body surface area involved (BSA) [6] in psoriasis patients. We also calculated the disease activity score 28 C reactive protein (DAS28-CRP) [7], bath ankylosing spondylitis disease activity Index (BASDAI) [8], swollen or tender joint counts, and involved joint counts in patients with psoriatic arthritis. The diagnosis of psoriatic arthritis was made based on CASPAR criteria [9]. The characteristics of the 44 psoriasis patients are summarized in Table 1.
| (n) | Psoriasis vulgaris (23) | Psoriatic arthritis (11) | Healthy controls (11) |
|---|---|---|---|
| Mean age | 52.3 (22-82) | 47.6 (27-63) | 42.3(28-67) |
| Sex(Male: Female) | 16 : 7 | 8 : 3 | 8:3 |
| Disease duration(years) | 11.3 (0.1-60) | 8.8 (3-40) | - |
| PASI | 12.2 (0.6–41.4) | 11.2 (0.3-44.1) | 0 |
| BSA | 22.7 (1-95) | 16.8 (1-95) | - |
| DAS28 | Not detected | 4.56 (2.80-6.48) | - |
| BASDAI | 4.81 (2.5-7.6) | - | |
| Swollen joint counts | 5.9 (0-14) | 0 | |
| Tender joint counts | 4.0 (1-14) | 0 | |
| Involved joint counts | 8.2 (1-23) | 0 |
Table 1. Clinical characteristics of psoriasis patients.
Skin biopsy specimens
Thirty-three 10% formalin-fixed and paraffin-embedded skin biopsy specimens of psoriasis (32 psoriasis vulgaris and 10 psoriatic arthritis) were prepared in this study. The controls consisted of eight each of human epidermal cyst and normal human epidermis.
Immunohistochemical staining
We prepared four-μm-thick tissue sections made from 10% formalin-fixed and paraffin-embedded psoriasis tissues and normal human skin in this study. Deparaffinized and rehydrated sections were treated with methanol with 0.3% hydrogen peroxidase for 15 min. After washing with 100 mM phosphate buffered saline (pH 7.4; PBS) for 5 min. The sections were incubated with VCP monoclonal antibody (1:500; Thermo Fisher, IL, USA) for 60 min at room temperature (RT). Then, VCP was incubated with ChemMate ENVISION reagent (Dako) for 30 min at RT. Finally, the sections were visualized with Stable DAB solution and counterstained with Mayer’s hematoxylin (Wako Pure Chemical, Osaka, Japan). The stainability was scored as 0=negative; 1=positive cells were scattered; 2=almost all cells were positive in the epidermis.
Reverse-phase protein array analysis
Reverse-phase protein array analysis (RPPA) was performed using almost the same Method as previously described by Kobayashi et al. with minor modifications [10].
Serum samples were diluted 1:100 with 0.01% TrironX-100/ PBS-and spotted onto ProteoChip glass slides (Proteogen, Seoul, Korea) in duplicate in a 640-spot/slide format using Glass Slide Microarrayer (V&P Scientific Inc., San Diego, CA, USA). The slides were immobilized in a moist chamber for 4 h at 37°C. After being blocked with 0.5% Casein sodium (Wako Pure Chemical Industries, Osaka, Japan) for 1 h at RT, the slides were incubated with 500-times diluted anti-VCP monoclonal antibody (Thermo Fisher Scientific, Rockford, IL, USA) for 1 h at RT. Then, the slides were incubated with 100- times diluted biotinylated anti-mouse IgG (BA-2000, Vector Laboratories, Burlingame, CA, USA) for 1 h at RT and 1,000- times diluted horseradish peroxidase-conjugated streptavidin (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) for 30 min at RT, respectively. The peroxidase activity was detected using 100-times diluted Cyanine 5-labeled Tyramide Signal Amplification System (PerkinElmer Life Sciences, Massachusetts, MA, USA) for 20 min at RT. The slides were counterstained with 2,000-times diluted Alexa Fluor 546- labeled goat anti human IgG (Life Technologies, Carlsbad, CA, USA) for 5 min at RT. Finally, the slides were scanned on a microarray scanner (Genepix 4000b, Molecular Devices, Sunnyvale, CA, USA). The fluorescence intensity, defined as the median net value of duplicate samples, was determined using the Genepix pro 6.0 software package (Molecular Devices).
Statistical analysis
Statistical analysis was performed using the Mann-Whitney U test. The area under the curve (AUC) and best cut-off point were calculated by employing receiver operating characteristic (ROC) analysis. Results were considered significant when P<0.05. All P-values used are two sided. Analysis was performed using StatFlex version 6.0 software (Artech Co., Ltd., Osaka, Japan).
Results
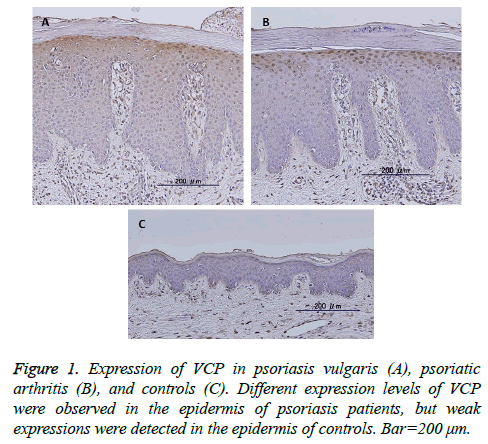
VCP expression was observed in the cytoplasm of the upper stratum spinosum and basal layer in the epidermis of 38 of 42 of psoriatic skin samples, including psoriasis vulgaris and psoriatic arthritis (Figures 1A and 1B), but was negative in 16 controls except four (Figure 1C). Even when positive, the expression levels were stronger in the psoriatic epidermis than in controls (P<0.01). No significant differences between the expression levels or localization of these proteins and clinicopathological features such as sex, age, psoriasis vulgaris vs. psoriatic arthritis, PASI score, BSA, DAS28, BASDAI, swollen or tender joint counts, and involved joint counts.
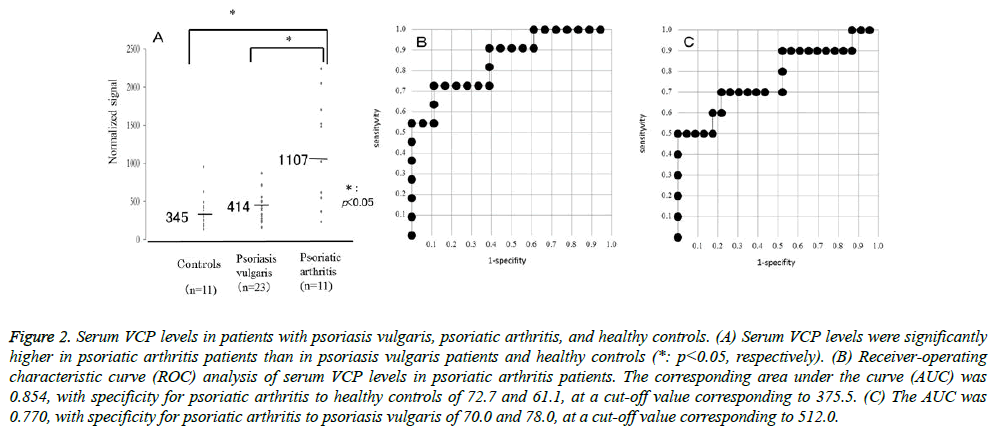
The serum levels of VCP were detected by RPPA in sera from psoriasis patients and healthy controls. VCP levels were significantly higher in psoriatic arthritis patients than in psoriasis vulgaris patients or healthy controls (P=0.0098, P=0.0058, respectively). Relative values of serum VCP levels in patients with psoriasis vulgaris, psoriatic arthritis, and healthy controls ranged from 156.5 to 873.5 (median: 413.9),234.5 to 2241.5 (median: 1107.3), and 137.5 to 956 (median: 344.5), respectively (Figure 2A). The AUC of the ROC curve between psoriatic arthritis patients and healthy controls was 0.854 (Figure 2B). When an optimal cut-off value of 375.5 for VCP was applied, the diagnostic sensitivity and specificity for psoriatic arthritis to healthy controls were 72.7 and 61.1, respectively. The AUC of the ROC curve between psoriatic arthritis and psoriasis vulgaris was 0.770 (Figure 2C). When an optimal cut-off value of 512.0 for VCP was applied, the diagnostic sensitivity and specificity for psoriatic arthritis to psoriasis vulgaris were 70.0 and 78.0, respectively. The serum VCP level was weakly correlated with the PASI score or BSA in patients with psoriasis vulgaris (P<0.05), but not in those with psoriatic arthritis. Also, the serum VCP level was not correlated with DAS28 or BASDAI in patients with psoriatic arthritis.
Figure 1: Serum VCP levels in patients with psoriasis vulgaris, psoriatic arthritis, and healthy controls. (A) Serum VCP levels were significantly higher in psoriatic arthritis patients than in psoriasis vulgaris patients and healthy controls (*: p<0.05, respectively). (B) Receiver-operating characteristic curve (ROC) analysis of serum VCP levels in psoriatic arthritis patients. The corresponding area under the curve (AUC) was 0.854, with specificity for psoriatic arthritis to healthy controls of 72.7 and 61.1, at a cut-off value corresponding to 375.5. (C) The AUC was 0.770, with specificity for psoriatic arthritis to psoriasis vulgaris of 70.0 and 78.0, at a cut-off value corresponding to 512.0.
Discussion
VCP is also called transitional endoplasmic reticulum (ER) ATPase and is known to be involved in a large number of independent cellular processes, which especially govern critical steps in ubiquitin-dependent protein quality control [11]. VCP plays a key role in ER-associated degradation, as reviewed in detail elsewhere [12]. Importantly, the degradation of both ER luminal and membrane proteins requires VCP, whose function in extracting substrates from the ER is essential for their delivery to the proteasome. Consequently, VCP inactivation elicits the unfolded protein response [13], which can trigger an ER-stress-induced apoptosis. The unfolded protein response is also activated during epidermal keratinocyte differentiation in psoriasis [14].
Beg et al. reported that nuclear factor kappa B (NFκB) was a transcription factor that, upon stimulation, translocates into the nucleus and triggers the expression of genes that promote cell proliferation and protect cells against apoptosis [15]. VCP has been proposed to be critically involved in the dissociation and proteasomal degradation of the inhibitor of NFκB IκBα [16]. Therefore, the up-regulation of VCP may result in the downregulation of IκBα and, consequently, in a hyperactive NFκB signaling pathway promoting cell proliferation [17]. On the other hand, abnormal VCP levels led to increased serum interleukin-6 (IL-6), tumor necrosis factor alpha (TNFα), and epidermal growth factor (EGF) levels and VCP caused cytokine imbalance and inflammation in organs [18]. Psoriasis is a chronic inflammatory skin disease, and so NF-kB, TNFα, and IL-6 play crucial role in the pathogenesis of psoriasis [19]. VCP may be associated with the pathogenesis of psoriasis because it causes abnormal keratinocyte differentiation and cytokine imbalance and leads to skin inflammation toward psoriasis. Our previous study showed that the serum IgG level of anti-VCP autoantibody was 5.4-times higher in patients with psoriasis vulgaris than those in healthy controls by twodimensional immunoblotting.
However, no anti-VCP autoantibody was detected in patients with psoriatic arthritis [5]. Furthermore, no serum IgG levels of VCP autoantibody or VCP levels in individual psoriasis patients were examined. The present study showed that serum VCP levels were significantly higher in patients with psoriatic arthritis than in those with psoriasis vulgaris or controls using the RPPA method with individual serum samples. Different results may be caused by differences in the methodology and numbers of cases. Another serum marker, STIP-1, which we previously reported, may be a useful serum diagnostic marker of psoriatic arthritis. The sensitivity and specificity of STIP-1 in psoriatic arthritis are the same as those of VCP [5]. In conclusion, our results suggest that VCP is a novel and differential sero-diagnostic marker of psoriatic arthritis.
Acknowledgements
The authors received no specific funding for this work and declare no conflict of interest.
References
- Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J InvestigDermatolSympProc 2004; 9: 136-9.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361: 496-509.
- Strober B, Teller C, Yamauchi P. Effects of etanercept on C-reactive protein levels in psoriasis and psoriatic arthritis. Br J Dermatol 2008; 159:322-330.
- Davidovici BB, Sattar N, Prinz J. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol 2010; 130: 1785-1796.
- Maejima H, Nagashiro R, Yanagita K. Moesin and stress-induced phosphoprotein-1 are possible sero-diagnostic markers of psoriasis. PLOS ONE 2014; 7: e1017773.
- Fredriksson T, Pettersson U. Severe psoriasis-oral therapy with a new retinoid. Dermatologica 1978; 157: 238-244.
- Paulus HE, Ramos B, Wong WK. Equivalence of the acute phase reactants C-reactive protein, plasma viscosity, and Westergren erythrocyte sedimentation rate when used to calculate American College of Rheumatology 20% improvement criteria or the Disease Activity Score in patients with early rheumatoid arthritis. Western Consortium of Practicing Rheumatologists. J Rheumatol 1999; 26: 2324-2331.
- Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994; 21: 2286-2291.
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54: 2665-73.
- Kobayashi M, Nagashio R, Jiang SX. Calnexin is a novel sero-diagnostic marker for lung cancer. Lung Cancer 2015; 90: 342-345.
- Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol 2012; 14: 117-123.
- Ye Y. Diverse functions with a common regulator: Ubiquitin takes command of an AAA ATPase. J StructBiol 2006; 156: 29-40.
- Kothe M, Ye Y, Wagner JS. Role of p97 AAA-ATPase in the retrotranslocation of the cholera toxin A1 chain, a non-ubiquitinated substrate. J Bio Chem 2005; 280: 28127-28132.
- Sugiura K, Muro Y, Futamura K. The unfolded protein response is activated in differentiating epidermal keratinocytes. J Invest Dermatol 2009; 129: 2126-2135.
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 1996; 274: 782-784.
- Asai T, Tomita Y, Nakatsuka S. VCP (p97) regulates NF-kappaBsignaling pathway, which is important for metastasis of osteosarcoma cell line. Jpn J Cancer Res 2002; 93: 296–304.
- Braun RJ, Zischka H. Mechanisms of Cdc48/VCP-mediated cell death-from yeast apoptosis to human disease. Biochimica et BiophysicaActa 2008; 1783: 1418-1435.
- Dec E, Rana P, Katheria V. Cytokine profiling in patients with VCP-associated disease. ClinTranslSci 2014; 7: 29–32.
- Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF. NF-kB: An essential transcription factor in psoriasis. J DermatolSci 2013; 69: 89-94.