ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 2
Using the differential adhesion method to isolate and culture mesenchymal stem cells and endothelial progenitor cells from rat bone marrow
| Lin Fang, Mingjuan Du, Yan Li, Lu Lin, Chen Zhang, Zhenmin Zhao* Department of micro-invasive plastic surgery, Plastic Surgery Hospital of Chinese Academic Medical Science and Peking Union Medical College, Beijing 100144, PR. China |
| Corresponding Author: Zhenmin Zhao,Department of cleft lip and palate surgery, Peking Union Medical College, PR. China. |
| Accepted: January 20, 2016 |
Bone Marrow Mesenchymal Stem Cells (BMMSCs) are adult stem cells with the capacity for selfrenewal and multilineage differentiation. Endothelial Progenitor Cells (EPCs) play a fundamental role in post-natal vascular repair. Both BMMSCs and EPCs are rare in bone marrow and therefore optimizing isolation and culture is required before they can be applied as parts of scientific research and clinical therapies. The aim of this study was to develop an efficient method to isolate and expand both BMMSCs and EPCs in the same rat bone marrow at the same time. In this study, rat BMMSCs and EPCs were isolated by differential adhesion method and cultured in vitro. Four passaged BMMSCs were CD29+, CD90+ and CD45-. EPCs were CD34+ and FLK-1+. BMMSCs differentiate in vitro into chondrogenic and adipogenic cells based on the positive staining with Alcian Blue, Type 2 Collagen and Oil Red O. In conclusion, the differential adhesion method was found to be a convenient and reliable method for the isolation and culture of both BMMSCs and EPCs from one bone marrow donor sample at one time.
Keywords |
||||||||
| Bone marrow mesenchymal stem cells, Endothelial progenitor cells, The differential adhesion method, Isolation. | ||||||||
Introduction |
||||||||
| Human adult mesenchymal stem cells (MSCs) were first identified by Friedenstein et al. when observing a certain population of cells that developed into fibroblastic colony forming cells (CFU-F) [1]. Ever since, the therapeutic uses and clinical applications of these cells have increased research interests in this field. However, depending upon the cell surface antigen, these cells are found to be different [1-3]. A wide range of non-hematopoietic stem cells exists in the bone marrow and MSCs are merely a subset of this population. These include “Bone marrow mesenchymal stem cells” (BMMSCs), which could be differentiated into cells with mesenchymal lineages such as osteoblasts, chondrocytes and adiopocytes [2,3] and “endothelial progenitor cells” (EPCs), which have great potential to promote angiogenesis. Among these investigated cells, BMMSCs attracted a lot of attention, since they have high ‘ex-vivo’ expansion potential, ability to differentiate into multiple lineages, immunomodulatory property and paracrine action [4-6]. On the basis of this, there are emerging clinical applications of the BMMSCs, including tissue repair, treatment of organ failure, autoimmune diseases and degenerative diseases [4-6]. With great angiogenic potential of EPCs, investigators are exploring the use of EPCs as possible new treatments for patients with cardiovascular diseases, cerebrovascular diseases, wound healing and limb ischemic diseases [7,8]. In recent years, both BMMSCs and EPCs are important cell sources for cell therapy, which has emerged as an innovative and effective approach for treating patients with various diseases [9,10]. The cell-based treatment performed primarily using autologous stem/progenitor cells may represent a hope for the cure of these diseases. Nevertheless, the cell numbers of BMMSCs and EPCs in the bone marrow are too low to satisfy the need of basic and clinical cell therapy [11]. Thus, the characterization/isolation of the stem cell subpopulations represents a major challenge to improve the efficacy of cell therapy. An efficient method to isolate and expand the cells in vitro is essential. | ||||||||
| In this study, we investigated the differential adhesion method to isolate and culture the BMMSCs and EPCs in the rat bone marrow from one bone-marrow sample at the same time. This could increase the utilization ratio of one bone marrow sample. | ||||||||
Materials and Methods |
||||||||
Animals |
||||||||
| Two to three weeks old male Sprague-Dawley rats (Institute of Animal, Academy of Military Medical Sciences, Beijing, China) were used. This study was carried out in strict compliance with the recommendations of Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Chinese Academic Medical Science and Peking Union Medical College (Permit Number: 20060828001). | ||||||||
Isolation and culture of BMMSCs and EPCs from rat bone marrow |
||||||||
| BMMSCs and EPCs were derived from 2-3 weeks old male rats. Rats were euthanized by CO2 overdose followed by thoracotomy. Whole bone marrow aspirates were flushed from bilateral femora and tibiae with a syringe 18-gauge needle containing 1 mL heparin. The marrow cells were transferred to a sterile tube and mixed with 5 mL culture medium (Dulbecco’s Modified Eagle Medium-low glucose, L-DMEM, Hyclone, Logan, Utah, USA, supplemented with 15% fetal bovine serum, GIBCO, USA, BEIJING TIANTECH BIOTECHNO D409 The Development Building N° 12 Xinxi Road BEIJING, penicillin G [100 U/mL] and streptomycin [100 µg/mL], Amresco Inc., Solon, Ohio, USA). | ||||||||
| Cells were cultured at 37°C and 5% CO2 in humidified incubators (The same temperature and same CO2% used for all culturing). To separate BMMSCs and EPCs, the differential velocity adherent culture method was used. When replacing the culture medium after 48h, the culture dish was shaken gently, then the culture medium containing the red cells and EPCs was removed. The adherent cells were then washed twice in PBS and cultured in culture medium, and marked as ‘BMMSCs’. | ||||||||
| The removed culture medium containing red cells and EPCs was centrifuged at 2000 rpm for 5 min at 4°C. Then the cell pellet was resuspended in M199 medium (supplemented with 20% fetal bovine serum, VEGF [20 ng/mL], bFGF [10 ng/mL], PeproTech Inc., Rocky Hill, New Jersey, USA). The cells were plated into fibronectin-coated plates, which marked as ‘EPCs’. The medium for both cells was changed every 3 days. | ||||||||
Morphology |
||||||||
| BMMSCs and EPCs at each passage were examined by light microscopy. | ||||||||
Flow cytometry analysis |
||||||||
| BMMSCs of the fourth passage were examined for surface protein molecule expression by flow cytometry. Cells were stained using antibody CD29-FITC, CD90-APC, CD45-PE. All antibodies were purchased from Pacific Heights Blvd, San Diego, CA. Isotype control antibodies were IgG1-FITC and IgG2b-PE. (Science Center, Drive San Diego, CA, USA). The labeled cells were analyzed by flow cytometry on FACS Aria flow cytometer (BD, USA). | ||||||||
Chondrocyte differentiation |
||||||||
| BMMSCs of the fourth passage were plated and grown until they were 70%~80% confluent, followed by chondrocyte differentiation in DMEM low glucose, 0.1µM dexamethasone, penicillin G [100 U/mL] and streptomycin [100 µg/mL], 1 mM L-proline, 10 ng/ml and TGF-ß, 1% and ITS Plus Culture Supplement. The cells were cultured for 19 days and then washed and fixed in 4% paraformaldhyde. The cells were then stained with Alcian blue and analyzed by immunohistochemistry for Type 2 Collagen. | ||||||||
Adipocyte differentiation |
||||||||
| For adipocyte differentiation, 70%~80% confluent BMMSCs of the fourth passage were treated with adipogenic media containing DMEM high glucose, 2 µM insulin, 0.5mM indometacin, 0.1 µM dexamethasone, 10% fetal bovine serum, penicillin G [100 U/mL] and streptomycin [100 µg/ mL]. The cells were cultured for 18 days and then washed and fixed in 4% paraformaldhyde. The cells were then stained with Oil Red O, which specifically stains lipid droplets. | ||||||||
Immunohistochemistry |
||||||||
| The cultured EPCs were immunohistochemically stained for CD34 and FLK-1. Briefly, the cells were cultured and grown until 80%~90% confluency. Then cells were washed with PBS and treated with methanol at -20°C for 20 minutes. The dishes were washed with PBS 3 more times. Rabbit anti-rat antibodies against CD34 and FLK-1 were used. | ||||||||
| The cells were incubated with the antibodies at 37°C for 1 hour. To remove the unbound antibody, the culture dishes were gently shaken at room temperature and washed 3 times with PBS. | ||||||||
| A goat anti-rabbit FITC-IgG was added to the dishes. Incubation and rinsing with PBS were performed as described above for the primary antibodies. The cells were then photographed. | ||||||||
Results |
||||||||
Cell morphology |
||||||||
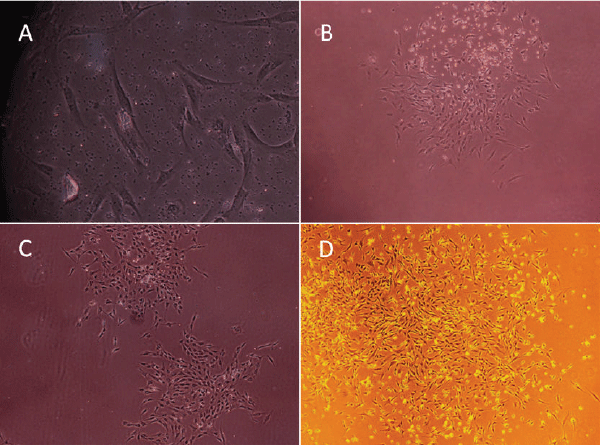
| BMMSCs: BMMSCs were spindle-shaped, attached to the culture dish tightly when plated to the dishes for 36 hours, and proliferated in the culture medium (Figure 1A). The cells gathered to form clusters in some area (Figure 1B). Fibroblastlike cell colonies formed in 4-5 days (Figure 1C). On day 5-10, the growth of cells was fast, colonies gradually expanded in size with the adjacent ones interconnected to each other. Some colonies even reached confluency. Spindle or triangle-like cells grew in size. On day 12, cells were radial or spiral shaped and reached confluency to from a single layer (Figure 1D). | ||||||||
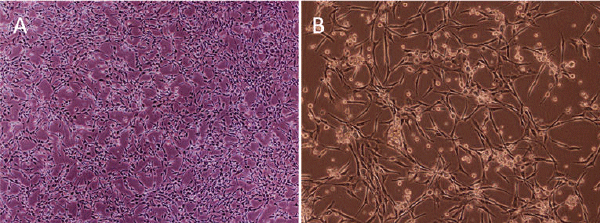
| EPCs: Only a few EPCs initially seeded on fibronectin-coated plates were adherent with a spindle-like shape. Cell colonies formed on day 5, with round cells in the center surrounded by spindle-like cells. Cell number grew gradually and tubular structures appeared (Figure 2A and 2B). | ||||||||
Cell characterization (the 4th passage cells) |
||||||||
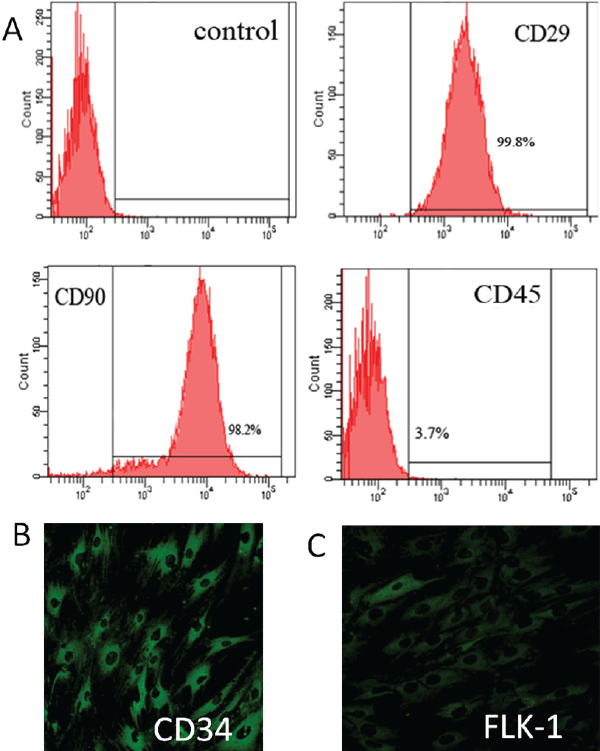
| To characterize BMMSCs, their phenotype was examined via flow cytometry. BMMSCs were positive for CD29 and CD90, which is considered as a marker for MSCs, and devoid of hematopoietic lineage marker CD45 (Figure 3A). To characterize EPCs, their phenotype was examined via immunofluorescence. Hematopoietic marker CD34 and FLK-1 were present on most of EPCs (Figure 3B and 3C). | ||||||||
Multipotential Differentiation |
||||||||
Chondrocyte differentiation |
||||||||
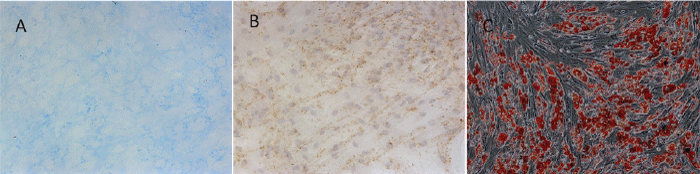
| BMMSCs are multipotential mesenchymal stem cells. Chondrogenic differentiation can be induced in vitro by treating cells with dexamethasone. To assess the chondrogenic capacity of BMMSCs, cells were stained with Alcian Blue. Marrow-derived MSCs form cell nodules associated with a well-organized ECM rich in Type 2 Collagen and sulfated proteoglycans [12]. These sulfated proteoglycans can be specifically detected using the stain Alcian Blue. Cultured BMMSCs resulted in the formation of dense nodules consistent with chondrogenic differentiation. The nodules were associated with an Alcian Blue-positive ECM, indicative of the presence of sulfated proteoglycans within the matrix (Figure 4A). Cells were also positive of Type 2 Collagen (Figure 4B). | ||||||||
Adipocyte differentiation |
||||||||
| For adipogenic differentiation, BMMSCs were cultured in the presence of 2µM insulin, 0.5mM indometacin and 0.1µM dexamethasone. After 18 days, the cells were stained with Oil Red O, and the lipid droplet accumulation was clearly detected in the cells (Figure 4C). | ||||||||
Discussion |
||||||||
| The bone marrow (BM) is an invaluable source of adult pluripotent stem cells, including hematopoietic stem cells (HSCs), EPCs, and MSCs. The therapeutic potential of BMMSCs for applications such as tissue engineering and gene therapy is enormous [13,14]. Investigators demonstrated that these cells could be differentiated in vitro into cells with mesenchymal lineages such as adiopocytes, chondrocytes, myoblasts and osteoblasts [2]. In various studies BMMSCs have proven to be an effective therapeutic agent in experimental models of tissue injuries [4-6]. EPCs contribute to reendothelialisation and neovascularisation. Evidence suggests EPCs are mobilized from the bone marrow into the peripheral blood in response to tissue ischemia or injury [15, 16]; these cells migrate to sites of damaged endothelium and differentiate into endothelial cells (ECs) [17], thereby improving blood flow and tissue repair. However, traditional bone marrow procurement procedures may be painful, frequently requiring general or spinal anesthesia and may yield low numbers of BMMSCs and EPCs upon processing. BMMSCs only account for a very small ratio of the bone marrow and it has been estimated that 1/10000 to 1/100000 of the bone marrow nuclear cells are BMMSCs [18,19]. EPCs only account for 1% of bone marrow mononuclear cells [20]. And these cells decline with age. Thus, from a practical standpoint, it is particularly important to establish an easy and feasible method for both MSCs and EPCs isolation, purification and propagation. | ||||||||
| In 1976, Friedenstein et al. established a simple and feasible method for in vitro MSC isolation and culture, which has been widely applied in the actual practice. However, there are still difficult in harvest plenty of MSCs for basic and clinical research. There is a need for long-time in vitro amplification and this procedure will affect the results of research. Common methods for isolating MSCs include whole marrow direct adherence, density gradient centrifugation, flow cytometry and immunobead methods. Flow cytometry and immunobead methods are considered as high cost and difficulty technique [21]. The extensive application of these methods is unreasonable for animal experiment. Common methods for isolating MSCs include whole marrow direct adherence and density gradient centrifugation. Density gradient centrifugation refers to the extraction of mononuclear cells for adherent culture according to the proportion of mononuclear bone marrow cells. Whole marrow direct adherence refers to regular culture medium changing to remove non-adherent cells based on the stem cell adherence characteristic to achieve MSC purification. Direct adherence is simple and convenient, obtains more MSCs than density gradient centrifugation and has an appropriate cell density for growth in culture flasks. In addition, MSCs purity increases after medium changes. Direct adherence can be regarded as the preferential method for MSC isolation [21,22]. On the other hand, EPCs play an important role in post-natal vascular repair and maintenance of vascular homeostasis through re-endothelialization and neovascularization [23]. However, there are also challenges to harnessing EPCs for cell therapy. One of these is their rarity (0.01-0.02 per 106 mononuclear cells), which makes EPC isolation challenging. Optimization of the cultivation and amplification of EPCs is therefore required before these cells may be appropriately investigated for use in clinical therapies. Nevertheless, there’s not much research so far on how to get both BMMSCs and EPCs from the same bone marrow sample at a time. To propagate a large number of primary BMMSCs and EPCs rapidly have become a prerequisite for these cells study and application. | ||||||||
| The differential adhesion method to isolate BMMSCs and EPCs according to their different adhesive velocity from rat bone marrow. This method is very useful, especially for animal research, which makes full use of the same bone marrow sample of small animal. Rats are most common used experimental animals because it is easy and economical to keep them. Many researchers isolate rMSCs or rEPCs from rats bone marrow obtained from the cancellous bone removed from the femur and tibia. It is a big problem for scientists to find a simple and practicable way to get more rMSCs or rEPCs within a shorter time. In this study, we used the differential adhesion method to isolate and culture the BMMSCs and EPCs from one rat bone marrow` Differential adhesion is a method, which can be used to separate cells of different types by way of their physical disparity to adhere to the plastic substrate of the cell culture plate. It takes 4-48 hours for BMMSCs and 2-3 hours for fibroblasts to adhere to the plate [21-24]. Based on this, we waited 4 hours to suspend and still the cells after the isolation to separate the fibroblasts and BMMSCs. EPCs don’t adhere to the plate easily, so as to separate the BMMSCs and EPCs, we waited 48 hours to change the medium and to plate EPCs present in the BMMSCs into fibronectin-coated culture plates. Time of change of the culture medium is essential in differential adhesion method. We found that 48 hours is most appropriate. If we change the medium at 24h, clusters of BMMSCs were decreased (data not shown). One reason could be that at that time adhered cells were unstable. If we chose to change the medium at 72 hours, we were not able to harvest enough EPCs from the medium (data not shown). | ||||||||
| Our results showed that BMMSCs constituted a rapidly expanding population of spindle-like or fibroblast-like cells. EPCs seeded on fibronectin-coated culture plates were round. The cell number grew gradually and tubular structures appeared. BMMSCs were positive for stromal cell-associated markers CD29 and CD90 and negative for hematopoietic lineage marker CD45, while EPCs were positive for hematopoietic marker CD34 and FLK-1. BMMSCs are capable of multipotential differentiation. Here we tested the potential of BMMSCs for chondrogenic and adipogenic differentiation. Cultured BMMSCs resulted in the formation of dense nodules that were positive for Alcian Blue. Cells also tested positive with Type 2 Collagen. For adipogenic differentiation, BMMSCs were stained with Oil Red O, which came out positive. These results indicated that BMMSCs differentiated toward chondral and adipose phenotypes [2-3]. | ||||||||
| In this study, we obtained both r-BMMSCs and r-EPCs from one bone marrow sample using simple and convenient methods of isolation and culture. Generally speaking, using the differential adhesion method the abundant and reliable r- BMMSCs and r-EPCs sources can be easily provided for animal experimentation in which a large number of cells are needed for cell therapy. | ||||||||
Figures at a glance |
||||||||
|
||||||||
References |
||||||||
|