ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 2
Turkish validation of National Institutes of Health (NIH) patient-reported outcomes measurement information system (PROMIS®) Gastroesophageal Reflux Disease (GERD) scale.
| Burak Özşeker1, Necdet Fatih Yaşar2, Muzaffer Bilgin3, Yasemin Kurt4, Huseyin Balcıoğlu4, Uğur Bilge4* 1Department of Internal Medicine Division of Gastroenterology, Muğla Sıtkı Koçman University 2Department of General Surgery, Eskişehir Osmangazi University 3Department of Biostatistics, Eskişehir Osmangazi University 4Department of Family Medicine, Eskişehir Osmangazi University |
| Corresponding Author: Uğur Bilge,
Department of Family Medicine,
Faculty of Medicine,
Eskisehir Osmangazi University, Turkey |
| Accepted: January 30, 2016 |
Aim: In this study; our aim was to validate the Turkish version of National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS®) Gastroesophageal Reflux Disease (GERD) Scale which will be the first Turkish validated GERD scale.
Material and methods: The GERD scale was translated into Turkish by three researchers, and a consensus meeting was held after the translation process. The Turkish text on which the researchers agreed was translated into English by an independent professional translator. As a result of this process, the researchers obtained the final version on which they agreed and the accuracy of which was proved by back translation.
Results: The questionnaire was administered to the patients with symptoms of GERD and complaints continuing for at least one week. A total of 106 patients (42 men and 64 women with an average age of 41.69 ± 13.69 years) took part in this study. Turkish version of the instrument was found to be quite reliable.
Conclusion: Turkish version of PROMIS-GERD scale is valid and reliable. We believe that this scale may be used for the objective assessment of GERD patients in clinical practice.
Keywords |
||
| Gastroesophageal reflux disease, Scale, Turkish validation. | ||
Introduction |
||
| Gastroesophageal Reflux Disease (GERD), a common disorder in the gastrointestinal system, deteriorates the quality of life of patients and may cause esophagitis and other complications [1,2]. Retrosternal burning and regurgitation are the main symptoms of this chronic disease that develops due to the regurgitation of acid from stomach to esophagus. Although international guidelines indicate that the diagnosis of GERD should be based on symptoms, there is no clear definition regarding the frequency of symptoms [3]. | ||
| The prevalence of GERD varies according to the population in which studies were conducted. While reflux symptoms ranged between 20% and 40% in the United States and Europe [1-3], a study conducted in Turkey suggests that the prevalence of GERD is 33.9% [4-6]. In addition to invasive methods, many other methods have been used for the diagnosis of GERD. Scales are one of these methods. Scales have been developed for an objective assessment of symptoms. The scales related to GERD generally question the frequency, severity, and in some cases, the effects on the life quality of symptoms. Among the significant advantages of GERD are that they are easily applicable, based on self-reporting, used for the purpose of screening, cost-effective and applicable to patients that present to healthcare centers due to symptoms of GERD [7,8]. | ||
| In 2004, the National Institutes of Health (NIH) set up the Patient-Reported Outcomes Measurement Information System (PROMIS®) in order to provide a public source that allows the assessment of illness experience on self-reports of patients. The PROMIS instruments are appropriate for both traditional and electronic ways of data collection. The PROMIS instruments-valid, reliable and easily applicable-allow users to establish common-language benchmarks for symptoms and to identify clinical thresholds. In gastroenterology, various types of users, including patients, providers, investigators, and regulators may use these instruments as guidance for clinical decision-making, clinical research and drug approval issues. Although over 100 PROs have been developed related to GI symptoms, there is still a need to develop standardized, detailed and electronic PRO measurement instruments, covering all GI symptoms, for clinical and research purposes [9]. | ||
| The Gastroesophageal Reflux (GER) item, one of the three domains associated with the foregut, was developed to assess four aspects of related symptoms: | ||
| • Sensations associated (reflux, regurgitation) or unassociated (lump in the throat) with food intake | ||
| • Painful sensations (heartburn, chest pain, throat burn) | ||
| • Belching gas (burping)/hiccups. | ||
| The GER items measure the frequency, severity, impact of and bother caused by these symptoms during the past 7 days [9,10]. Our aim was to validate the Turkish version of PROMISGERD scale which will be the first Turkish validated GERD scale. | ||
Material and Methods |
||
PROMIS GERD scale |
||
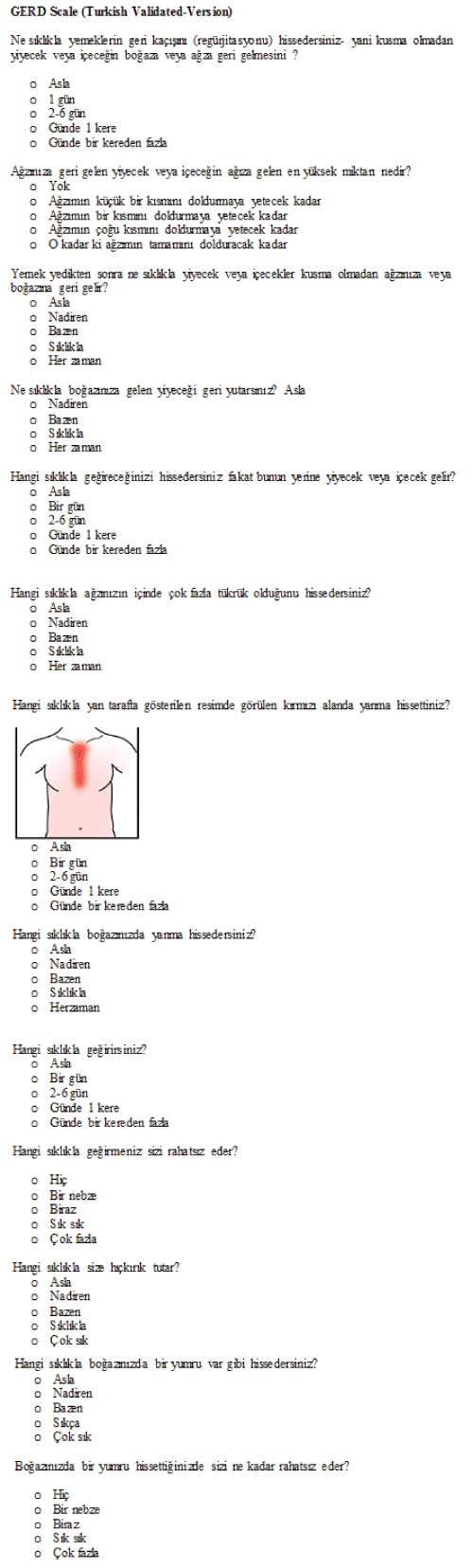
| PROMIS, developed by NIH, provides patient-reported outcome measures for the assessment of diseases, including GI disorders. The PROMIS GERD, one of the eight categories in the PROMIS GI item banks, is designed to measure the frequency, severity, impact of and bother caused by main GERD symptoms (especially heartburn and regurgitation), based on the assumption that the complaints continue for at least seven days. PROMIS GERD scale has 13 items [9,10]. | ||
| The questionnaire consisted of 13 questions, and in each question, the answers were scored from 0 to 4. The points for each question were summed. According to the scoring system in the original questionnaire, 0 point refers to not symptomatic, 1-3 points to least symptomatic, 4-7 points to mild symptomatic, 8-15 points to moderately symptomatic and 16 and more points to most symptomatic. The questionnaire was administered to the patients that presented to Gastroenterology Policlinic of Muğla University and Family Medicine Polyclinic of Osmangazi University with symptoms of GERD and complaints continuing for at least one week. Sociodemographic data were collected from the patients before the administration of questionnaire. | ||
Translation process |
||
| The GERD scale was translated into Turkish by three researchers, and a consensus meeting was held after the translation process. The Turkish text on which the researchers agreed was translated into English by an independent professional translator. After the researchers compared the English text, a product of back translation, and the source text in terms of meaning and comprehensibility, they decided that there were no differences between the two texts. As a result of this process, the researchers obtained the final version on which they agreed and the accuracy of which was proved by back translation. | ||
| In order to test reliability of the questionnaire form, Cronbach’s alpha coefficient was calculated for each question. In the case that Cronbach’s alpha coefficient was minimum 0.70, it was considered that the questions were consistent with each other [11]. | ||
Patient collection |
||
| This study was conducted in the policlinics of Family Medicine at Osmangazi University Faculty of Medicine and Gastroenterology at Muğla Sıtkı Koçman University Faculty of Medicine between June and August 2015. The scale was administered to the patients that presented to the policlinics with reflux complaints lasting for at least one week. Ethical approval was received from Muğla Sıtkı Koçman University Faculty of Medicine to carry out this study. | ||
Results |
||
| A total of 106 patients (42 men and 64 women with an average age of 41.69 ± 13.69 years) took part in this study. The average GERD scale score was 21.40 ± 8.92. The results indicate that 1 person was not symptomatic, 6 persons were mildly symptomatic, 20 persons were moderately symptomatic and 79 persons fell under the category of most symptomatic. The Cronbach’s alpha coefficient of the scale was 0.783, which indicates that the Turkish version of the instrument was quite reliable. The distribution of Cronbach’s alpha values by questions are provided in table 1. | ||
Discussion |
||
| The PROMIS-GERD scale consists of short and easily applicable 13 questions that allow us to assess each GERD symptom individually. In addition to heartburn and regurgitation, i.e. the most common symptoms of GERD, NIH PROMIS GERD scale enables us to measure the severity of other GERD symptoms [9,10,12]. The scale comprises 13 questions addressing main GERD symptoms, including heartburn frequency, heartburn severity, heartburn bother, throat burn frequency, regurgitation frequency, regurgitation bother, ‘wet burp’ frequency, and night time awakening because of regurgitation. The NIH PROMIS consortium conducted 3 focus group studies (with 28 participants with GERD) in order to develop these items and cognitive interviews in order to refine the findings [9]. | ||
| After identifying 45 GERD-related items in the NIH PROMIS item bank, the researchers refined these items to obtain the final 13-item GERD instrument, which is valid in clinical practice and efficient in terms of applicability [9,10]. GERD symptoms are highly common in the society. The severity of symptoms is higher among people that specifically present to healthcare centers due to GERD, compared to GP and other GI patients. Among the factors that play a role in the severity of symptoms are visceral anxiety, marital status, education and GI comorbidities [1-3,9,10]. This study shows that the Turkish version of PROMIS-GERD scale is valid and reliable. We believe that this scale may be used for the objective assessment of GERD patients in clinical practice. | ||
Tables at a glance |
||
|
||
Figures at a glance |
||
|
||
References |
||
|