ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Health Science and Bio Convergence Technology: Edition-II
TNFAIP8 protein expression in pancreatic associated diseases and its effect on the apoptosis and invasion of human pancreatic cancer PANC-1 cells
1Department of General Surgery, the Affiliated Hospital of North China University of Science and Technology, PR China
2Department of Emergency Medicine, the Affiliated Hospital of North Polytechnic University, PR China
- *Corresponding Author:
- Guozhi Zhang
Department of General Surgery
The Affiliated Hospital of North China University of Science and Technology, PR China
Accepted date: April 17, 2017
Objective: To observe the expression in tissue of pancreatic related diseases, and to investigate the effect of TNFAIP8 on the apoptosis and invasion of pancreatic cancer PANC-1 cell.
Methods: RT-PCR method for detection of TNFAIP8 expression in normal pancreatic tissue, pancreatitis and pancreatic cancer tissues; electroporation transfection method of transfecting TNFAIP8 siRNA into PANC-1 cells, Western blot method for the expression of TNFAIP8 in PANC-1 cells after TNFAIP8 siRNA transfected; flow cytometry and Transwell to detect the apoptosis and invasion ability changes of PANC-1 cells after silencing TNFAIP8.
Results: The expression level of TNFAIP8 in pancreatic cancer tissues was (2.83 ± 0.38), which was significantly higher than that of normal pancreatic tissue (0.98 ± 0.12) and pancreatitis (1.26 ± 0.28) (P<0.01); after using RNA interference method to silence TNFAIP8 expression level in PANC-1 cells of pancreatic cancer, apoptosis level of PANC-1 cell increased from (5.83 ± 1.54%) to (28.92 ± 6.29%) (P<0.01), and the invasive ability of PANC-1 cells was significantly decreased (P<0.01).
Conclusion: High expression of TNFAIP8 in pancreatic cancer tissues may promote the invasiveness of pancreatic cancer cell and inhibit the apoptosis of pancreatic cancer cells, involving in the occurrence and development of pancreatic cancer.
Keywords
Tumor necrosis factor-α-induced protein, Pancreatitis, Pancreatic cancer, Apoptosis, Invasion.
Introduction
Pancreatic cancer is a highly malignant tumor of digestive system, which is the fourth cause of diseases in patients died with cancer, and in recent years the incidence of pancreatic cancer has presented increasing trend year by year [1]. The main clinical manifestations of pancreatic cancer patients are obstructive jaundice, upper abdominal or back pain, weight loss and anorexia, etc. Although the 1 year and 5 years’ survival rates of patients with pancreatic cancer have increased obviously, the prognosis of patients with pancreatic cancer is still not ideal [1,2]. Currently the pathogenesis of pancreatic cancer is not fully understood. The tumor alpha necrosis factorinduced protein 8 (TNFAIP8) family is a tumor necrosis factor- α-induced protein (tumor necrosis factor-αTNF-α) found in recent years [3,4]. TNFAIP8 family members include TNFAIP8, TIPE1 (TNFAIP8L1), TIPE2 (TNFAIP8L 2) and TIPE 3 (TNFAIP8L 3). The transfection of TNFAIP8 expression plasmid in breast cancer cell elevated the levels of TNFAIP8 expression in breast cancer cells, and proliferation and invasion ability of breast cancer cell was significantly higher [5]. After silencing the expression of TNFAIP8 in tumor cells, the invasion ability of tumor cell decreased significantly [6], which indicated TNFAIP8 had an important role in occurrence and development of tumor. However, the function of TNFAIP8 in pancreatic carcinoma is rarely reported previously. Therefore, the goal of this study is to observe the expression of TNFAIP8 in pancreatic carcinoma, and to investigate the effect of TNFAIP8 on the apoptosis and invasion of PANC-1 cells, which is a kind of pancreatic carcinoma cell. The results are reported as follows.
Materials and Methods
Clinical data
30 cases of patients’ tissues with pancreatic cancer confirmed by clinical diagnosis and pathological examination from August 2012 to December 2013 in our hospital were collected, aged 48-69 years old with average age (58.14 ± 15.62); at the same time 30 cases of patients’ tissue specimens with chronic pancreatitis and 10 normal pancreatic tissues were collected in same hospital and same period with the age of patients with chronic pancreatitis 42-71 years old and average age (55.41 ± 17.26) years old; normal control age was 45-70 years old with average age (52.94 ± 16.82) years old.
Material and reagent
TNFAIP8 antibody was purchased from Cell Signaling Technology, Co; horseradish peroxidase labeled Goat antimouse IgG was purchased from Snata Cruz, Co; CytoBuster protein extraction kit was from Novagen company; protease inhibitors were from Thermo company; TNFAIP8 siRNA was purchased from Ambion company; electroporation transfection reagent was purchased from Lonza company; transwell for invasion was purchased from invasion; Gibco company.
Method
RT-PCR TNFAIP8: Upstream primer: 5'- TGAAGATGGAGCACTGCTGA-3', downstream primer: 5'- GGTCTGTTACCCGTTAGGAAG-3'. PCR reaction conditions: 95°C 5 min, 95°C 10 s, 60°C 20 s with a total of 40 cycles. At the same time amplification of GAPDH was used as internal control. 3 cervical cancer tissues were grinded into powder in liquid nitrogen, total RNA was extracted by Trizol method, and the expression amount of TNFAIP8 tissue was calculated by RT-PCR method. The calculation method of TNFAIP8 expression was found in the literature [7].
Transfection steps and transfection efficiency detection: Before transfection with cells were washed with PBS for 2 times and cells to be transfected were put in room temperature with 100 μL in advance for Nucleofector electroporation resuspending; cell suspension was added with 300 pmol/ sample TNFAIP8 siRNA; the above cell suspension was transferred into the electric cup, and covered with electric rotary cup cover; choose corresponding electric rotary programs, and put the electric cup into electroporator before operating electric program; immediately take electric cup after electric transfer procedure ; use suction pipette provided by the kit to absorb 500 μL preheating medium into the electric cup, and gently transfer cells into 12 well plates; put cells in a CO2 incubator at 37°C, and change culture medium after 6 h; the transfection efficiency detection could be conducted by flow cytometry in positive control 8~12 h after transfection.
Western blot: After equivalent extraction of cell separated by protein by 10% SDS-PAGE separation gel and 5% concentration gel with semi dry transferred to the nitrocellulose filter membrane, and closed with TBST containing 5% BSA at room temperature for 2 h, adding first antibody incubated overnight at 4°C. On the second day, use 0.1% TBST to wash membrane for 3 times, each time for 5 min, adding the second antibody marked by HRP markers at room temperature for 1 h. After 0.1% TBST washing membrane, the nitrocellulose membrane striped the color with Supersignal West Femto HRP sensitive chemiluminescent substrate. Actin was used as internal control. All the experiments were repeated at least 3 times.
Detection of apoptosis: Cells were collected and centrifuged, 1000X g for 5 min and washed by PBS containing 1% BSA for 1 time. The cells were resuspended with 100 μl Binding buffer solution, adding 2 μl Annexin V dye and 0.1 L V PI dye with avoiding light for 15 min, and washing for 2 times with PBS containing 1% BSA.
Transwell cell invasion assay: After cell transfection for 48 h, count with 0.25% trypsin digestion cell. Cell suspension with concentration of 5 × 105 was prepared from serum-free RPMI1640 culture medium. RPMI1640 culture medium containing 10% fetal bovine serum was added in the lower layer of Transwell chamber, and cells were placed on upper layer of chamber after paving under the condition of 7°C and 5% CO2 for 24 h. Take the chamber out and the fixedly stain the cells in the lower chamber with 0.1% crystal violet to calculate the number of cell invasion.
Statistical methods: Use SPSS 16.0 software, t test or ANOVA test method for statistical analysis of the data and P<0.05 for statistically significant difference.
Results
Expression of TNFAIP8 in pancreatic cancer tissues
The expression level of TNFAIP8 in normal tissues was (0.98 ± 0.12), and the expression level in pancreatitis tissues was (1.26 ± 0.28). The expression level in pancreatic cancer tissue was (2.83 ± 0.38), which was significantly higher than that of normal tissue and pancreatitis tissues (P<0.01).
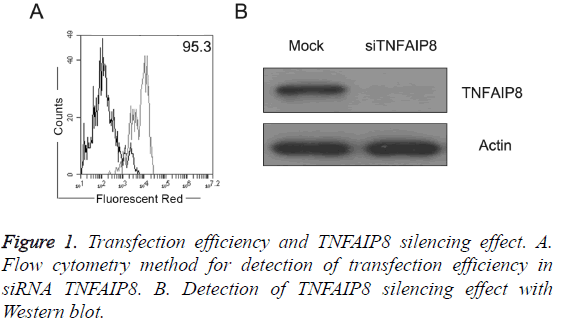
Detection of transfection efficiency and TNFAIP8 silencing effect
As shown in Figure 1A, pancreatic cancer PANC-1 cells were RNA transfected by Lonza power transfer method with transfection efficiency reaching more than 90%; after transfection of TNFAIP8 siRNA, TNFAIP8 expression level in PANC-1 cells was significantly lower (Figure 1B).
The apoptosis change of PANC-1 cells after silencing TNFAIP8
The level of cell apoptosis in control group was (5.83 ± 1.54%), and after TNFAIP8 expression in PANC-1 cells, the apoptosis level of PANC-1 cell was (28.92 ± 6.29%). After silencing TNFAIP8, apoptotic level of PANC-1 cell was significantly increased (P<0.01).
The invasion ability change of PANC-1 cell after silencing TNFAIP8
After silencing TNFAIP8 expression in PANC-1 cells, PANC-1 cell invasion ability was significantly reduced (P<0.01).
Discussion
Tumor necrosis factor-α-induced protein (tumor alpha necrosis factor--induced protein 8, TNFAIP8) is the first widely discovered and studied TNFAIP8 family member. Tumor necrosis factor alpha (tumor necrosis factor-αTNF-α) can increase expression level of TNFAIP8 on the surface of cells through the activation of NF-kB pathway [8,9]. TNFAIP8 was found to express in a variety of in tumor cells, such as K562, MOLT4, A549, SKOV and SW480 [3]. Studies showed that compared to the healthy control group, the mRNA protein expression level of TNFAIP8 in lung cancer tissues was significantly increased. This study also found that compared to the healthy control group and pancreatitis tissues groups, the mRNA protein expression level of TNFAIP8 in pancreatic cancer tissues was significantly increased (P<0.01). The results were consistent with those reported in the literature. It is known that TNFAIP8 can play a regulatory role in apoptosis of cells through the expression of the known apoptosis protein Caspase-8 and Caspase-3 [10]. And upregulating TNFAIP8 in breast cancer cells can increase the proliferation and invasion ability of breast cancer cells, meanwhile, silencing the expression of TNFAIP8 can inhibit the proliferation and invasion ability of breast cancer cells. These results suggested that TNFAIP8 could be used as an oncogene playing an important role in the development and progression of cancer. The effect of TNFAIP8 on proliferation and invasion of pancreatic cancer cells has rarely been reported in the literature. Therefore, this study used electroporation transfection method of transfecting TNFAIP8 siRNA into PANC-1 cells, and observed the effect of TNFAIP8 on apoptosis and invasion ability after silencing TNFAIP8 expression. The results showed that compared to apoptosis level of the control group(5.83 ± 1.54%), after the silence of TNFAIP8 expression in PANC-1 cells, PANC-1 cell apoptosis level was(28.92 ± 6.29%), suggesting that after TNFAIP8 silencing, PANC-1 cell apoptosis level increased significantly (P<0.01). At the same time, this study also found that after silencing the expression of TNFAIP8 in PANC-1 cells, invasion of PANC-1 cells significantly reduced (P<0.01). In conclusion, the results of this study suggested that high expression of TNFAIP8 in pancreatic cancer tissues may promote the invasiveness of pancreatic cancer cell and inhibit the apoptosis of pancreatic cancer cells, involving in the occurrence and development of pancreatic cancer. However, the regulation mechanism of TNFAIP8 on the apoptosis and invasion ability of pancreatic cancer cells still needs further and deep study.
References
- Vincent A, Herman J, Schulick R. Pancreatic cancer. Lancet 2011; 378: 607-620.
- Sharma C, Eltawil KM, Renfrew PD. Advances in diagnosis, treatment and palliation of pancreatic carcinoma. World J Gastroenterol 2011; 17: 867-897.
- Kumar D, Whiteside TL, Kasid U. Identification of a novel tumor necrosis factor-alpha-inducible gene, SCC-S2, containing the consensus sequence of a death effector domain of fas-associated death domain-like interleukin- 1beta-converting enzyme-inhibitory protein. J Biol Chem 2000; 275: 2973-2978.
- Patel S, Wang FH, Whiteside TL. Identification of seven differentially displayed transcripts in human primary and matched metastatic head and neck squamous cell carcinoma cell lines: implications in metastasis and/or radiation response. Oral Oncol 1997; 33: 197-203.
- Kumar D, Gokhale P, Broustas C. Expression of SCC-S2, a antiapoptotic molecule, correlates with enhanced proliferation antumorigenicity of MDA-MB 435 cells. Oncogene 2004; 23: 612-616.
- Zhang C, Chakravarty D, Sakabe I. Role of SCC-S2 in experimental metastasis and modulation of VEGFR-2, MMP-1, and MMP-9 expression. Mol Ther 2006; 13: 947-955.
- Zou R, Hu Z, Chen H. The expression and significance of miR-199a in cervical cancer and cervical intraepithelial neoplasia. J Med Res 2011; 40: 55-59.
- Horrevoets AJ, Fontijn RD, van Zonneveld AJ. Vascular endothelial genes that are responsive to tumor necrosis factor-alpha in vitro are expressed in atherosclerotic lesions, including inhibitor of apoptosis protein-1, stannin, and two novel genes. Blood 1999; 93: 3418-3431.
- You Z, Ouyang H, Lopatin D. Nuclear factor-kappa B-inducible death effector domain-containing protein suppresses tumor necrosis factor-mediated apoptosis by inhibiting caspase-8 activity. J Biol Chem 2001; 276: 26398-26404.
- Laliberte B, Wilson AM, Nafisi H. TNFAIP8: a new effector for Galpha (i) coupling to reduce cell death and induce cell transformation. J Cell Physiol 2010; 225: 865-874.