ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 3
The value of contrast-enhanced computed tomography in evaluating patient response to icotinib for non-small cell lung cancer
Jinping Yu1, Haixia Liu2*, Hong Chen3, Shuhua Ren4
1Department of Pharmacy, Branch of Tangshan Worker’s Hospital, Tangshan 063000, PR. China
2Department of Radiology, Tangshan Worker’s Hospital, Tangshan 063000, PR. China
3Department of Chemoradiotherapy, Tangshan Worker’s Hospital, Tangshan 063000, PR. China
4Department of Thoracic Surgery, Tangshan Worker’s Hospital, Tangshan 063000, PR. China
Accepted date: March 03, 2016
We aimed to evaluate the value of contrast-enhanced Computed Tomography (CT) imaging in analyzing the therapeutic effects of icotinib for Non-Small Cell Lung Cancer (NSCLC). Forty-five patients with histologically proven NSCLC underwent contrast-enhanced CT scanning before and after therapy with icotinib. All the data were automatically analyzed, and changes in each parameter before and after therapy were compared. According to the therapeutic response based on the Response Evaluation Criteria in Solid Tumors and 3-month follow-up evaluations, 26 of 45 patients received effective treatment and 19 patients who received ineffective treatment. The mean density and enhancement extent of lesions were not statistically different before treatment in patients who received effective treatment or in those who received ineffective treatment (P>0.05). After treatment, the mean density and enhancement extent of lesions for the 26 patients who received effective treatment were significantly reduced as compared to their respective pretreatment values (P=0.000). No significant difference was detected in any parameter before and after treatment for the 16 patients who received ineffective treatment (P>0.05). The mean density and enhancement extent of lesions in patients who received effective treatment were significantly decreased as compared to those who obtained ineffective treatment (P<0.05). In addition to size changes, tumor density, the degree of enhancement, and cavity changes found on contrast-enhanced CT scans were each associated with treatment response. Contrast-enhanced CT scans have significant value in determining the effect of icotinib in NSCLC patients.
Keywords
Contrast-enhanced CT, Non-small-cell lung cancer, Icotinib, Effect evaluation.
Introduction
Cancer is the leading cause of death in economically developed countries and the second leading cause of death in developing countries [1]. The incidence of lung cancer has increased over time, and it has become the leading cause of cancer-related deaths worldwide. The 5-year survival rate is less than 15%, and lung cancer results in more than 1.4 million deaths annually, despite significant advances in both diagnostic and therapeutic approaches [2-4]. Lung cancer is a heterogeneous disease that has historically been divided into two main types based on differing disease patterns and treatment strategies: Small Cell Lung Carcinoma (SCLC) and Non-Small Cell Lung Cancer (NSCLC) [4]. NSCLCs account for approximately 80% of lung cancers, and they show remarkable heterogeneity considering the histology, pathogenesis, prognosis, and response to treatments [5]. NSCLCs comprise three major histological subtypes: Adenocarcinoma (AD), Squamous Cell Carcinoma (SCC), and Large Cell Carcinoma (LCC) [6]. The molecular characterization of lung tumors has greatly influenced current treatment strategies; however, this has not resulted in a significant extension of survival of lung cancer patients to date.
It is important to note that combination chemotherapy regimens using a platinum doublet result in a median overall survival of 8-11 months [7]. Clinical outcomes in patients with NSCLC continue to be poor, with an overall survival of 7 to 9 months and an objective response rate of less than 10% [8]. Therefore, the search for more effective and safe treatment methods for lung cancer is an important area of research. Recent advances in molecular characterization assist in stratifying NSCLC patients according to their potential benefit from targeted therapies. Icotinib (Conmana®, Zhejiang Beta Pharma Inc., Hangzhou, China) is a small-molecule Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitor (TKI) with a chemical structure and active mechanism similar to gefitinib and erlotinib; icotinib was recently approved by the State Food and Drug Administration of China [9,10]. Icotinib is a molecular targeted therapy drug, with the characteristics of high selectivity and low toxicity. It is an effective drug for the treatment of advanced NSCLC [11,12], and it has received considerable attention from domestic and foreign researchers; however, there is no consensus about the efficacy of icotinib [10]. NSCLCs respond to targeted therapies differently than they respond to conventional chemotherapy. NSCLC patients with mutations in the gene coding for the EGFR tyrosine kinase domain achieve response rates greater than 70% and superior progression-free survival when treated with an EGFR TKI as compared to standard chemotherapy [13]. We did not attempt to use a new assessment method to replace the traditional Response Evaluation Criteria in Solid Tumors (RECIST) [14]; however, the RECIST only evaluates the change in tumor size and thus has limitations [15]. Each of the type of lung cancers therefore requires its own individualized treatment response criteria [16]. With targeted therapies, intratumoral morphologic changes may occur, such as cavitation, hemorrhage, and/or necrosis, which usually develop as a response to the chemotherapeutic agent and may lead to tumor growth inhibition [15]. Icotinib treatment leads to a unique spectrum of response patterns and drug-specific toxic effects, some of which may be detected via imaging, such as morphology, attenuation, size, and structure. Thus, when exploring imaging changes, it is important to use simple and effective detection methods to guide treatment and evaluate its effects.
Computed Tomography (CT) is commonly used for the initial diagnosis; segmentation of pulmonary structures; image registration; and disease detection, classification, and quantification of patients with NSCLC [17]. New developments in CT scanning have the potential to provide improved anatomical and functional assessments of lung cancers, resulting in more individualized and targeted therapy. Advanced CT scanners permit a high-resolution, comprehensive evaluation of the entire chest in a single breath hold lasting several seconds; they have an improved radiation dose profile that generates an isotropic dataset that allows detailed anatomical assessment as well as functional assessment of lung cancers [18,19]. Contrast-enhanced CT scanning is the most commonly used method for detecting thoracic tumors and has the greatest diagnostic value. Although contrast-enhanced CT is considered conventional, we expect lower expenditures and for patients to be exposed to fewer radiation doses while providing more information not only on the tumor size but also on changes in internal structures. The purpose of the current study was to evaluate tumor responses to icotinib treatment by retrospectively analyzing contrastenhanced CT images before and after treatment in 45 patients who received icotinib (Conmana).
Material and Methods
Patient eligibility
The inclusion criteria were as follows: eligible patients were diagnosed with NSCLC and were treated with icotinib in Tangshan Worker’s Hospital between January 2012 and June 2013; complete clinical and imaging data was available; and NSCLCs were clinical stages IIIb-IV before treatment. Tumors were graded according to the International Association for the Study of Lung Cancer version 7 NSCLC tumor-node-metastasis clinical staging standard. In addition, contrast-enhanced chest CT was performed 1 week before and 1 month after treatment, to detect the measurable primary tumor; the survival time was 3 months, with 3 months of follow-up with chest CT examinations. The exclusion criteria were as follows: a small primary tumor; routine mediastinal windows (window width 400 HU, window level 30 HU); display was difficult to detail; a primary tumor that could not be measured; and any other treatment such as radiofrequency ablation, radiotherapy, and chemotherapy. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Tangshan Worker’s Hospital in China. The approval number is GRYY-LL- 2013-38. Written informed consent was obtained from all participants.
Patient treatment
All patients were treated with icotinib hydrochloride tablets (Zhejiang Beta Pharma Inc., Hangzhou, China). Patients consumed the icotinib tablets orally, at a dose of 125 mg (three times a day) for 28 days. The drug was continued after 28 days until progressive disease, death, or intolerable toxicity was documented.
CT examination
Chest CT scans were obtained for the 45 patients treated in the expansion arm of the study at two time points: 1 week before the icotinib tablets were taken and 1 month after treatment. For imaging, we used a 64-detector row CT scanner (Philips Medical Systems, Best, the Netherlands). Acquisition parameters were as follows: tube voltage of 120 kVp, reference tube current-time product of 280 mAs, temporal resolution of 0.6 s, total scan time of 30 s, in-plane spatial resolution of 0.625 × 0.625 mm, slice thickness of 5 mm, and image matrix size of 512 × 512. The scanning range was from the apex to the base of the lung, and the collecting diameter was 360 mm. A high-pressure syringe was used for forearm venous injection of the contrast agent, and the injection speed was 5 mL per second. The scan delay time was 25 seconds after the administration of the contrast material (100 mL of 300 mg/mL ioversol; Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) in the arterial phase and 60 seconds in the parenchymal phase. Mediastinal window axial images were selected to measure the maximum diameter and lesion density before and after lesion enhancement, with the Hounsfield Unit (HU) as a unit of measurement; the maximum possible number of lesions was measured in multiple patients. The imaging software was used to segment Regions of Interest (ROIs). A circular ROI occupying half to two-thirds of the area was defined in the center of each mass to measure precontrast and postcontrast CT attenuation values (calculated via subtraction of the CT attenuation value on the nonenhanced image from that of the enhanced image; arterial and parenchymal phase CT attenuation values, respectively). While defining ROIs, special attention was placed on excluding calcified areas. To avoid measurement errors, two chest radiologists with experience in thoracic CT interpretation evaluated CT images and manually drew tumor ROIs for measurement. The enhancement degree was calculated by subtracting CT attenuation values on nonenhanced images from those on enhanced images (arterial and parenchymal phase attenuation values minus the precontrast CT attenuation values, respectively). The degree of enhancement (HU) during different phases of the enhanced CT examination was classified as “high” if it was >30 HU and “low” if it was ≤ 30 HU as compared to the precontrast CT attenuation value. The tumor density was defined as the CT attenuation value as measured on unenhanced CT images.
Evaluation standard of treatment response
For anticancer drugs, the RECIST [14] is the objective evaluation standard for the curative effect in solid tumors. The responses in this case were divided into Complete Remission (CR), Partial Remission (PR), Stable Disease (SD), and Progressive Disease (PD). Patients with CR and PR were classified as the remission group, while those with SD and PD were classified as the non-remission group. According to the RECIST, the sum of the longest diameter of the target lesions (SLD) is calculated, and the four response categories are defined as follows: CR (complete disappearance of all lesions, confirmed at ≥ 4 weeks), PR (a ≥ 30% decrease in SLD from baseline, confirmed at ≥ 4 weeks), PD (a ≥ 20% increase in SLD from the smallest SLD), and SD (neither PR nor PD). Patients were not assessed only once, rather, regular follow-up chest CT examinations continued for more than 3 months.
Statistical analysis
Numerical variables are expressed as mean ± standard deviation. CT parameters and tumor sizes were compared before and during treatment using a t-test. All calculations were performed using SPSS v17.0 software (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was regarded statistically significant for all the tests.
Results
Baseline data
Forty-five cases were evaluable, and cytological diagnoses were AD (n=21), SCC (n=9), and other types of NSCLC (n=15). The patients were aged 42-81 years, with an average age of 53.3 ± 10.2 years. Twenty-seven patients were women, while 18 were men. Twenty-six patients comprised the remission group, and 19 patients comprised the non-remission group. Patient age, gender, performance status, clinical stage, and pathological type had better equilibrium and were more comparable in the two groups before treatment as compared to after treatment (P>0.05; Table 1). Before treatment, the mean tumor size in the remission group was 40.6 ± 23.1 mm, and the mean tumor size in the non-remission group was 41.8 ± 28.8 mm; there was no significant difference in the tumor size between the two groups (P>0.05; Table 2). After treatment, the mean tumor size in the remission group decreased to 14.3 ± 10.3 mm, and the mean tumor size in the non-remission group increased to 52.3 ± 31.3 mm; the difference in the tumor size between the two groups was significant (P=0.001; Table 3).
| Group | Cases | Gende, n(%) | PS Score, n(%) | Stage,n(%) | Pathological type,n(%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | 1 | 2 | IIIb | IV | SC | Adenocarcinoma | Others | ||
| Remission group | 26 | 11(42) | 15(58) | 7(27) | 19(73) | 8(31) | 18(69) | 5(9) | 12(46) | 9(35) |
| None remission group | 19 | 8(42) | 11(58) | 5(26) | 14(74) | 6(44) | 13(56) | 4(21) | 9(47) | 6(32) |
| T value | - | 0 | 0.002 | 0.003 | 0.052 | |||||
| P value | - | 0.909 | 0.964 | 0.954 | 0.974 | |||||
Table 1: Comparison of baseline data of patients of two groups.
| Group | Tumor size (mm) | Plain Scan (Hu) | Arterial phase (Hu) | Parenchymal (Hu) phase |
|---|---|---|---|---|
| Remission group (n=26) | 40.6 ± 23.1 | 33.59 ± 7.98 | 60.85 ± 23.23 | 61.92 ± 20.23 |
| None remission group (n=19) | 41.8 ± 28.8 | 31.52 ± 6.42 | 66.21 ± 21.76 | 58.26 ± 22.00 |
| T value | 1.833 | 0.93 | -0.786 | 0.578 |
| P value | 0.183 | 0.357 | 0.436 | 0.566 |
Table 2: Comparison of tumor size and density of the two groups patients before treatment (x̅ ± s).
| Group | Tumor size (mm) | Plain Scan(Hu) | Arterial phase(Hu) | Parenchymal phase(Hu) |
|---|---|---|---|---|
| Remission group (n=26) | 14.3 ± 10.3 | 28.65 ± 5.07 | 53.04 ± 19.44 | 49.15 ± 13.56 |
| None remission group (n=19) | 52.3 ± 31.3 | 33.42 ± 7.13 | 66.79 ± 17.75 | 62.79 ± 13.31 |
| T value | 24.768 | -2.49 | -2.43 | -3.358 |
| P value | 0.001 | 0.018 | 0.019 | 0.002 |
Table 3: Comparison of tumor size and density of the two groups patients after treatment (x̅ ± s).
The mean tumor size of the remission group was significantly different before and after treatment (P<0.001; Table 4); however, no significant difference was detected in mean tumor size of the non-remission group before and after treatment (P>0.05; Table 5).
| Group | Tumor size (mm) | Plain Scan(Hu) | Arterial phase(Hu) | Parenchymal phase(Hu) |
|---|---|---|---|---|
| Before treatment (n=26) | 40.6 ± 23.1 | 33.59 ± 7.98 | 60.85 ± 23.23 | 61.92 ± 20.23 |
| After treatment (n=19) | 14.3 ± 10.3 | 28.65 ± 5.07 | 53.04 ± 19.44 | 49.15 ± 13.56 |
| T value | 5.447 | 6.524 | 6.424 | 5.54 |
| P value | 0.024 | 0 | 0 | 0 |
Table 4: Comparison of CT examination tumor size and density before and after treatment in the remission patients (x̅ ± s).
| Group | Tumor size (mm) | Plain Scan(Hu) | Arterial phase(Hu) | Parenchymal phase(Hu) |
|---|---|---|---|---|
| Before treatment (n=26) | 41.8 ± 28.8 | 31.52 ± 6.42 | 66.21 ± 21.76 | 58.26 ± 22.00 |
| After treatment (n=19) | 52.3 ± 31.3 | 33.42 ± 7.13 | 66.79 ± 17.75 | 62.79 ± 13.31 |
| T value | 0.223 | -1.764 | -0.292 | -1.16 |
| P value | 0.64 | 0.095 | 0.774 | 0.261 |
Table 5: Comparison of CT examination tumor size and density before and after treatment in the none remission patients (x̅ ± s).
CT examination
No significant difference was detected between both the groups considering the density and degree of enhancement before treatment (P>0.05; Table 2). The density and degree of enhancement of the remission group were significantly lower than those in the non-remission group after treatment (P<0.05; Table 3). The lesion density and degree of enhancement were significantly reduced in patients in the remission group after treatment (P=0.000; Table 4; Figures 1 and 2). In the remission group, some tumors changed into cavitary nodules with a definite decrease in size after treatment (Figure 2), and the density of some lesions was slightly increased (Figure 1). Lesion density and degree of enhancement changed in the non-remission group after treatment; however, this difference was not statistically significant (P>0.05; Table 5; Figure 3). Tables 4 and 5 show a comparison of the parameters before and after treatment in both the groups.
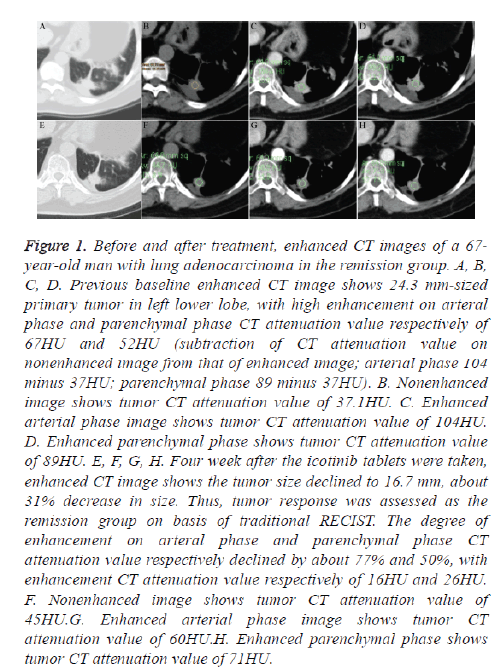
Figure 1: Before and after treatment, enhanced CT images of a 67- year-old man with lung adenocarcinoma in the remission group. A, B, C, D. Previous baseline enhanced CT image shows 24.3 mm-sized primary tumor in left lower lobe, with high enhancement on arteral phase and parenchymal phase CT attenuation value respectively of 67HU and 52HU (subtraction of CT attenuation value on nonenhanced image from that of enhanced image; arterial phase 104 minus 37HU; parenchymal phase 89 minus 37HU). B. Nonenhanced image shows tumor CT attenuation value of 37.1HU. C. Enhanced arterial phase image shows tumor CT attenuation value of 104HU. D. Enhanced parenchymal phase shows tumor CT attenuation value of 89HU. E, F, G, H. Four week after the icotinib tablets were taken, enhanced CT image shows the tumor size declined to 16.7 mm, about 31% decrease in size. Thus, tumor response was assessed as the remission group on basis of traditional RECIST. The degree of enhancement on arteral phase and parenchymal phase CT attenuation value respectively declined by about 77% and 50%, with enhancement CT attenuation value respectively of 16HU and 26HU. F. Nonenhanced image shows tumor CT attenuation value of 45HU.G. Enhanced arterial phase image shows tumor CT attenuation value of 60HU.H. Enhanced parenchymal phase shows tumor CT attenuation value of 71HU.
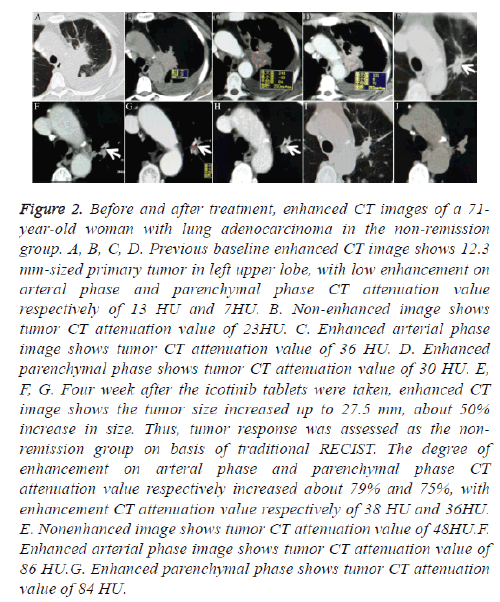
Figure 2: Before and after treatment, enhanced CT images of a 71- year-old woman with lung adenocarcinoma in the non-remission group. A, B, C, D. Previous baseline enhanced CT image shows 12.3 mm-sized primary tumor in left upper lobe, with low enhancement on arteral phase and parenchymal phase CT attenuation value respectively of 13 HU and 7HU. B. Non-enhanced image shows tumor CT attenuation value of 23HU. C. Enhanced arterial phase image shows tumor CT attenuation value of 36 HU. D. Enhanced parenchymal phase shows tumor CT attenuation value of 30 HU. E, F, G. Four week after the icotinib tablets were taken, enhanced CT image shows the tumor size increased up to 27.5 mm, about 50% increase in size. Thus, tumor response was assessed as the nonremission group on basis of traditional RECIST. The degree of enhancement on arteral phase and parenchymal phase CT attenuation value respectively increased about 79% and 75%, with enhancement CT attenuation value respectively of 38 HU and 36HU. E. Nonenhanced image shows tumor CT attenuation value of 48HU.F. Enhanced arterial phase image shows tumor CT attenuation value of 86 HU.G. Enhanced parenchymal phase shows tumor CT attenuation value of 84 HU.
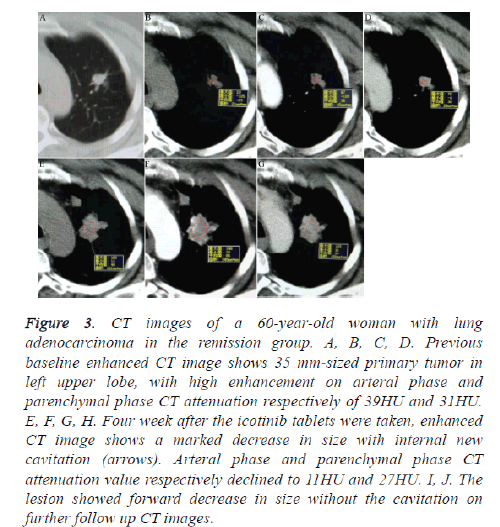
Figure 3: CT images of a 60-year-old woman with lung adenocarcinoma in the remission group. A, B, C, D. Previous baseline enhanced CT image shows 35 mm-sized primary tumor in left upper lobe, with high enhancement on arteral phase and parenchymal phase CT attenuation respectively of 39HU and 31HU. E, F, G, H. Four week after the icotinib tablets were taken, enhanced CT image shows a marked decrease in size with internal new cavitation (arrows). Arteral phase and parenchymal phase CT attenuation value respectively declined to 11HU and 27HU. I, J. The lesion showed forward decrease in size without the cavitation on further follow up CT images.
Discussion
The traditional RECIST represents the sole standard used to determine tumor response to chemotherapy. However, a few recent studies have demonstrated that RECIST cannot be used to precisely estimate tumor responses for targeted therapy, as this assessment involves only the use of the change in tumor size as its basic evaluation and thus has certain limitations [15,20]. Currently, new molecular targeted agents may be used as first-line treatment for select cancers. For example, for NSCLC, a selective EGFR TKI [4,21] has been found to extensively inhibit the growth of human tumor cell xenografts in nude mice by inhibiting angiogenesis [22]. Icotinib is a highly potent EGFR TKI that is orally administered [11]. Targeted agents induce intratumoral hemorrhage, necrosis, or cavitation rather than tumor shrinkage; morphological changes within a tumor have been emphasized as a type of tumor response, and thus, recent studies on tumor responses to targeted therapies have emphasized morphologic changes within a tumor [15,23]. In our study, many tumor changes were observed via contrast-enhanced CT. Tumor density was typically reduced due to necrosis after icotinib treatment; however, the densities of a few lesions slightly increased (Figure 1), seemingly owing to intratumoral hemorrhage [15]. We observed that some lesions showed cavitation (Figure 2). Cavity and size changes as well as the tumor attenuation value account for tumor responses to treatment [15]. The most common tumor responses observed in our study were changes in enhancement degree: an obvious decrease in enhancement degree after treatment was considered a tumor response (Figures 1 and 2). In contrast, because the enhancement degree obviously increased, treatment with icotinib was considered disappointing (Figure 3). These characteristic images of lung tumors can reflect histological changes in lesions accurately and dynamically, including the size, shape, density, and enhancement of features. The growth activity of lung cancer is reflected by angiogenesis and the formation of tumor feeding arteries, with tumor neovascularization acting as the physiological basis ensuring tumor nutrition, supply, metabolism, and proliferation [24].
Artery supply varies greatly among lung cancers and can be the result of multiple bronchial arteries, the pulmonary artery, or a doubled blood supply from the bronchial artery [25]. The type and size of the tumor as well as that of the new blood vessels responsible for the tumor blood supply were observed to be enhanced on CT examination of lung cancer. The degree of tumor enhancement was closely related to the microvascular number [26]. The findings of the present study reflect that icotinib could block signal transduction through the EGFR signaling pathway and inhibit tumor growth by blocking the nutrient supply to tumor cells and promoting tumor cell apoptosis. Lesion density and degree of enhancement increased slightly after treatment in the non-remission group. We believe that individual differences in histopathological characteristics and tumor tissue blood supply could affect the inhibitory effects of drugs on angiogenesis and tumor cell proliferation.
Simple, non-invasive CT can be used to quantitatively measure lung tumor density and degree of enhancement of internal structures before and after treatment, and could indicate changes in the internal tumor structure, angiogenesis, and tumor inhibition. CT can be used as an objective indicator to assess the curative effect of icotinib as a molecular targeted drug. Unfortunately, our study has several limitations. The methodology extracts CT lung lesion features based on size, and the ROI needs to be larger. This could be a potential limitation for the analysis of lung nodules, and future work will continue to determine the application of other methods for obtaining prognostic information for patients with NSCLC. The present study is designed to provide initial evidence of a relationship between CT-enhanced degree and short-term chemotherapeutic outcomes and as well as to demonstrate how tumor size, morphologic changes, attenuation, and structures may respond to treatment. Furthermore, this preliminary study is expected to lead to further research into the processes involved in multi-parameter and long-term outcomes of drugs.
Conclusion
Contrast-enhanced CT has significant value in determining the effect of icotinib in NSCLC patients. In addition to size changes, tumor density, enhancement degree, and cavity changes were associated with a response to treatment. Such results imply that CT allows for quantification of tumor enhancement, facilitating monitoring the internal morphologic changes of the tumor over time as well as a more accurate evaluation of the tumor response. As only a minority of patients benefit from targeted therapies, the identification of early imaging markers to predict treatment outcomes requires further investigation.
Ethical considerations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 [6]. Informed consent was obtained from all patients for being included in the study.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90.
- Colombi D, Di Lauro E, Silva M, Manna C, Rossi C, De Filippo M, Zompatori M, Ruffini L, Sverzellati N. Non-small cell lung cancer after surgery and chemoradiotherapy: follow-up and response assessment. Diagn Interv Radiol 2013; 19: 447-546.
- O'Brien ME. Lung cancer screening: Is there a future. Indian J Med Paediatr Oncol 2014; 35: 249-252.
- Wood SL, Pernemalm M, Crosbie PA, Whetton AD. Molecular histology of lung cancer: from targets to treatments. Cancer Treat Rev 2015; 41: 361-375.
- Markou A, Liang Y, Lianidou E. Prognostic, therapeutic and diagnostic potential of microRNAs in non-small cell lung cancer. Clin Chem Lab Med 2011; 49: 1591-1603.
- Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer 1995; 75: 191-202.
- Cappuzzo F, Ciuleanu T, Stelmakh L. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010; 11: 521-529.
- Hotta K, Fujiwara Y, Kiura K, et al. Relationship between response and survival in more than 50,000 patients with advanced non-small cell lung cancer treated with systemic chemotherapy in 143 phase III trials. J Thorac Oncol 2007; 2: 402-407.
- Shi B, Zhang XB, Xu J, Huang XE. Systematic Analysis of Icotinib Treatment for Patients with Non-Small Cell Lung Cancer. Asian Pac J Cancer Prev 2015; 16: 5521-5524.
- Guan YS, He Q, Li M. Icotinib: activity and clinical application in Chinese patients with lung cancer. Expert Opin Pharmacother 2014; 15: 717-728.
- Tan F, Shi Y, Wang Y, Ding L, Yuan X, Sun Y. Icotinib, a selective EGF receptor tyrosine kinase inhibitor, for the treatment of non-small-cell lung cancer. Future Oncol 2015; 11: 385-397.
- Zhao X, Zhu G, Chen H, Yang P, Li F, Du N. Efficacy of icotinib versus traditional chemotherapy as first-line treatment for preventing brain metastasis from advanced lung adenocarcinoma in patients with epidermal growth factor receptor-sensitive mutation. J Cancer Res Ther 2014; 10: 155-159.
- Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin 2011; 61: 91-112.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228-247.
- Kim YN, Lee HY, Lee KS. Dual-energy CT in patients treated with anti-angiogenic agents for non-small cell lung cancer: new method of monitoring tumor response. Korean J Radiol 2012; 13: 702-710.
- Tirumani SH, Fairchild A, Krajewski KM, Nishino M, Howard SA, Baheti AD, Rosenthal MH. Anti-VEGF molecular targeted therapies in common solid malignancies: comprehensive update for radiologists. Radiographics 2015; 35: 455-474.
- Ganeshan B, Abaleke S, Young RC, Chatwin CR, Miles KA. Texture analysis of non-small cell lung cancer on unenhanced computed tomography: initial evidence for a relationship with tumour glucose metabolism and stage. Cancer Imaging 2010; 10: 137-143.
- Lee WK, Lau EW, Chin K, Sedlaczek O, Steinke K. Modern diagnostic and therapeutic interventional radiology in lung cancer. J Thorac Dis 2013; 5: 511-523.
- Schmitz S, Rommel D, Michoux N, Lhommel R, Hanin FX, Duprez T, Machiels JP. Dynamic contrast-enhanced computed tomography to assess early activity of cetuximab in squamous cell carcinoma of the head and neck. Radiol Oncol 2015; 49: 17-25.
- Oliver TG, Patel J, Akerley W. Squamous non-small cell lung cancer as a distinct clinical entity. Am J Clin Oncol 2015; 38: 220-226.
- Zhao J, Xiong J. Advances on driver oncogenes of non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2015; 18: 42-47.
- Shi YK. Current status and perspectives of individualized therapy for non-small cell lung cancer based on molecular targeting. Zhonghua Zhong Liu Za Zhi 2012; 34: 398-400.
- Colombi D, Di LE, Silva M. Non-small cell lung cancer after surgery and chemoradiotherapy: follow-up and response assessment. Diagn Interv Radiol 2013; 19: 447-456.
- Tanigawa N, Amaya H, Matsumura M, Lu C, Kitaoka A, Matsuyama K, Muraoka R. Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res 1997; 57: 1043-1046.
- Kiessling F, Boese J, Corvinus C, Ederle JR, Zuna I, Schoenberg SO, Brix G. Perfusion CT in patients with advanced bronchial carcinomas: a novel chance for characterization and treatment monitoring. Eur Radiol 2004; 14: 1226-1233.
- Yamashita K, Matsunobe S, Takahashi R, Tsuda T, Matsumoto K, Miki H, Oyanagi H, Konishi J. Small peripheral lung carcinoma evaluated with incremental dynamic CT: radiologic-pathologic correlation. Radiology 1995; 196: 401-408.